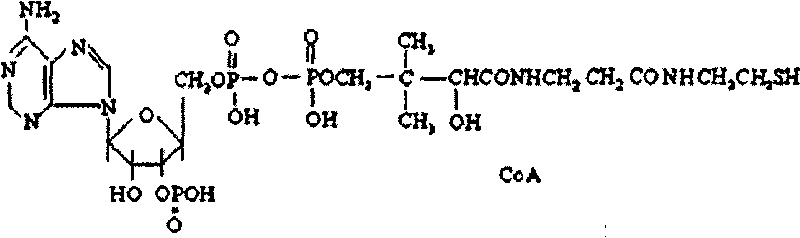
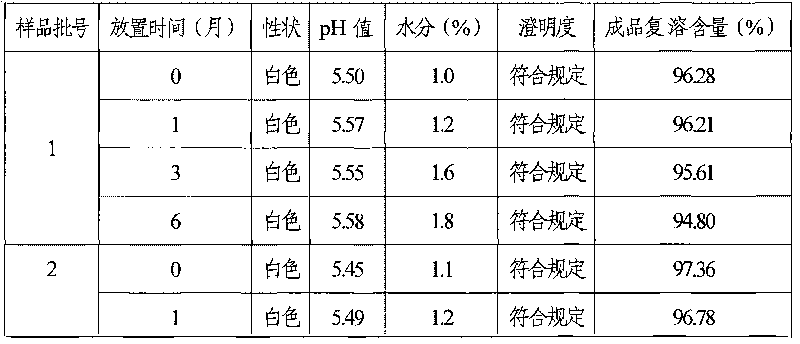
Coenzyme A medicament freeze-drying preparation and process for preparing same
A technology for freeze-dried preparations and coenzymes, applied in the field of medicine, can solve the problems of increased production cost, easy to exceed the internal control range, large changes, etc., and achieve the effect of stability assurance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] 1. Raw and auxiliary materials: 1 million units of coenzyme A; 100g of mannitol; 5g of cysteine hydrochloride; 10g of calcium gluconate; 20g of phosphate buffered saline;
[0025] 2. Preparation process:
[0026] 1. Preparation of liquid medicine
[0027] 1.1 Mannitol treatment: Weigh mannitol, add 2000mL water for injection into a stainless steel bucket and dissolve to obtain a solution ①.
[0028] 1.2 Treatment of cysteine hydrochloride and calcium gluconate: Weigh cysteine hydrochloride in a beaker, add 1000mL water for injection to dissolve to obtain a solution②; weigh calcium gluconate in a beaker, add 500mL water for injection, boil to dissolve to obtain a solution ③.
[0029] 1.3 Combine solution ①, solution ②, and solution ③, add 0.2% (weight volume percentage) activated carbon and stir for 20 minutes, then filter and decarbonize to obtain filtrate ④.
[0030] 1.4 Raw material processing: Weigh phosphate buffered saline, add 2000 ml of water for injecti...
Embodiment 2
[0050] Embodiment 2: except that the amount of raw and auxiliary materials is different, all the other are the same as embodiment 1.
[0051] Raw materials: coenzyme A 1.1 million units; mannitol 600g; cysteine hydrochloride 20g; calcium gluconate 20g; phosphate buffered saline 60g; add water for injection to 10000mL.
Embodiment 3
[0052] Embodiment 3: except that the amount of raw and auxiliary materials is different, all the other are the same as embodiment 1.
[0053] Raw materials: coenzyme A 900,000 units; mannitol 200g; cysteine hydrochloride 10g; calcium gluconate 5g; phosphate buffer saline 10g; add water for injection to 10000mL.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com