Oral care composition
An oral care and composition technology, applied in the field of oral care compositions, can solve problems such as disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
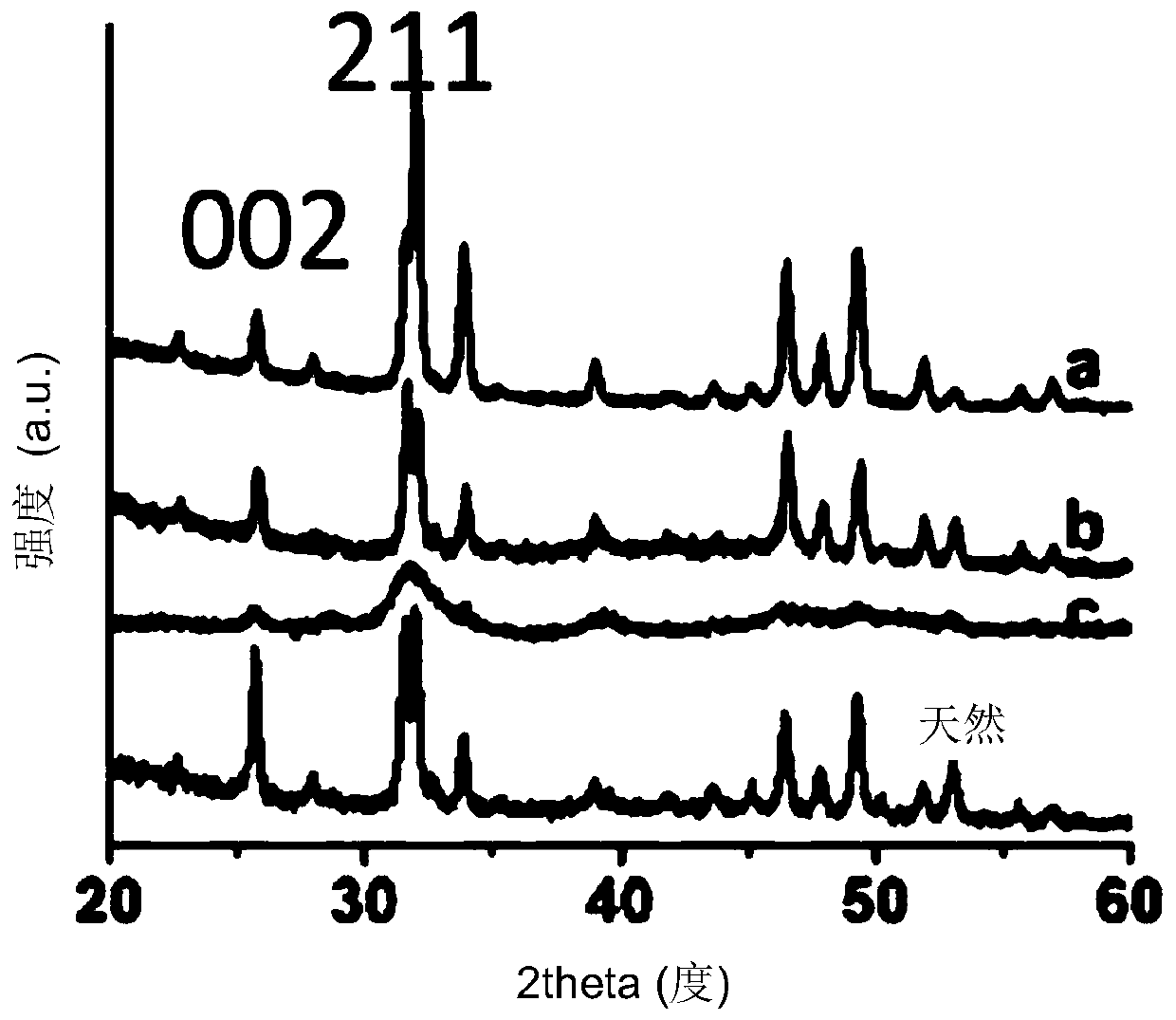
[0097] This example illustrates the effect of silicate sources on tooth enamel remineralization.
[0098] Preparation of remineralization solution
[0099] material
[0100] Unless specifically mentioned, all chemicals used in this study were purchased from Aladdin Reagent (Shanghai, China) and were of analytical grade. Use three times distilled water and filter all solutions through a 0.22μm Millipore membrane before use.
[0101] Remineralization solution
[0102] The remineralization solution is prepared by mixing equal volumes of calcium-containing solution (Ca solution) and phosphate-containing neutralized buffer solution (P solution). By dissolving the calculated amount of CaCl 2 And MgCl 2 ·6H 2 O, prepare calcium solution. By using Na 2 HPO 4 , KCl, NaCl, Na 2 SO 4 , NaHCO 3 , Glutamic acid (Glu), polyacrylic acid (PAA) and tris(hydroxymethyl)aminomethane (Tris, from Sigma-Aldrich, St. Louis, Missouri, USA) to prepare a phosphate-containing solution. Before mixing, the sodi...
Embodiment 2
[0114] This example demonstrates the effect of polyacrylic acid on the remineralization of tooth enamel.
[0115] Preparation of remineralization solution
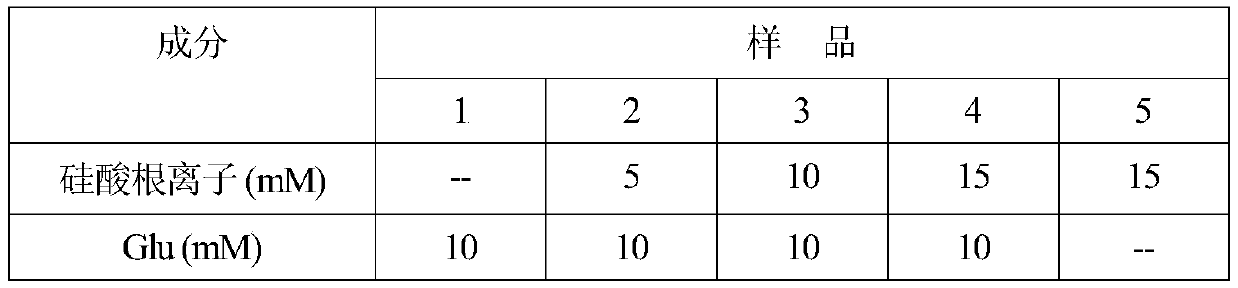
[0116] As described in Example 1, the same protocol was used to prepare the remineralization solution. After mixing, the final concentration of each substance in the remineralization solution is listed in Table 1. The concentrations of Glu, silicate ion and PAA in different samples are listed in Table 3.
[0117] table 3
[0118]
[0119] method
[0120] As described in Example 1, the same protocol was used to evaluate the remineralization of enamel.
[0121] result
[0122] After 6 days of treatment with different remineralization solutions, SEM images showed that new layers were formed on all enamel plates. For sample 6, in the absence of PAA, no ordered structure was observed on the enamel surface. For samples 7 and 8 containing PAA, it was shown that orderly flaky crystallites were formed. Moreover, as the concentration of...
Embodiment 3
[0125] This example demonstrates the effect of glutamic acid, silicate source and polyacrylic acid on the remineralization of tooth enamel.
[0126] Preparation of remineralization solution
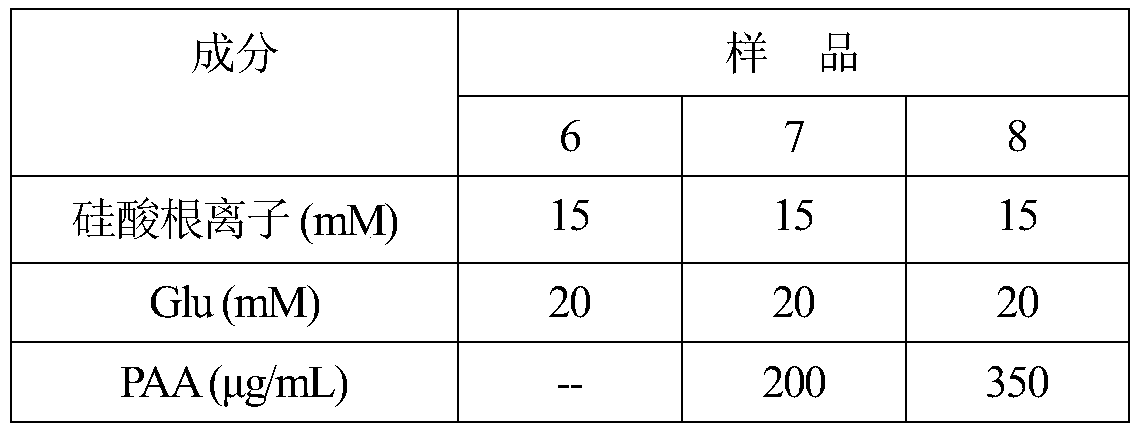
[0127] As described in Example 1, the same protocol was used to prepare the remineralization solution. After mixing, the final concentration of each substance in the remineralization solution is listed in Table 1. The concentrations of Glu, silicate ions and PAA in different samples are listed in Table 4.
[0128] Table 4
[0129]
[0130] method
[0131] As described in Example 1, the same protocol was used to evaluate the remineralization of enamel.
[0132] result
[0133] After 6 days of treatment with different remineralization solutions, SEM images showed that new layers were formed on all enamel plates. For sample 10 containing 10 mM Glu, no ordered structure was observed to be formed on the enamel surface. For samples 7 and 9 containing a higher concentration of Glu, it was shown that ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com