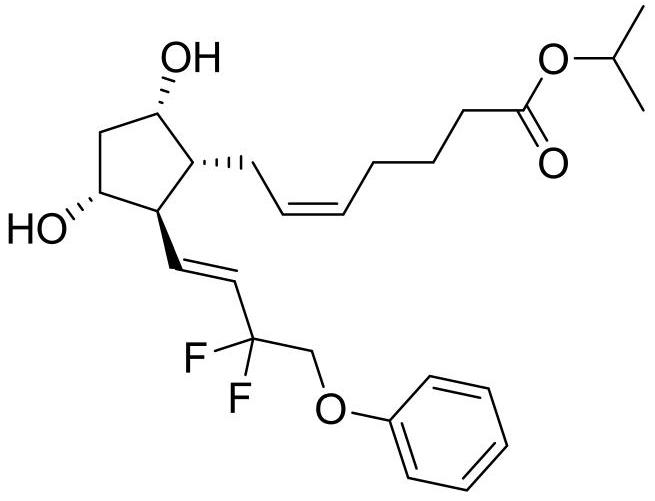
Eye drops for treating glaucoma and preparation method thereof
A technology for eye drops and glaucoma, which is applied in the field of medicine, can solve the problems of low solubility of tafluprost, poor water solubility and poor stability of tafluprost, and achieve the effects of reducing impurities, improving curative effect and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] An eye drop for treating glaucoma, its formula is:
[0028] Tafluprost 0.0375g
[0029] Modified polylysine 1.05g
[0030] Citric acid 1.25g
[0031] Water for injection 1000mL, adjust the pH to 6.7.
[0032] Preparation:
[0033] Step 1: at 10°C, weigh the amount of tafluprost in the formula, add water for injection, stir to form an emulsion, add modified polylysine, and form nano micelles after stirring;
[0034] Step 2: Dissolving the stabilizer citric acid with water for injection at 10°C to obtain an aqueous solution of the stabilizer citric acid;
[0035] Step 3: At 10°C, mix the aqueous solution of stabilizer citric acid with the micelles obtained in Step 1, then add water for injection, and stir to dissolve;
[0036] Step 4: Adjust the pH to 6.7, add sterilized water for injection to 1000mL, filter and fill, and sterilize at 125°C to obtain the finished preparation.
Embodiment 2
[0038] An eye drop for treating glaucoma, its formula is:
[0039] Tafluprost 0.040g
[0040] Modified polylysine 2.5g
[0041] Ascorbic acid 1.25g
[0042] Water for injection 1000mL, adjust the pH to 6.7
[0043] Preparation:
[0044] Step 1: at 10°C, weigh the amount of tafluprost in the formula, add water for injection, stir to form an emulsion, add modified polylysine, and form nano micelles after stirring;
[0045] Step 2: Dissolving the stabilizer ascorbic acid with water for injection at 10°C to obtain an aqueous solution of the stabilizer;
[0046] Step 3: At 10°C, mix the aqueous solution of the stabilizer with the micelles obtained in Step 1, then add water for injection, and stir to dissolve;
[0047] Step 4: Adjust the pH to 6.7, add sterilized water for injection to 1000mL, filter and fill, and sterilize at 125°C to obtain the finished preparation.
Embodiment 3
[0049] An eye drop for treating glaucoma, its formula is:
[0050] Tafluprost 0.0425g
[0051] Modified polylysine 2.75g
[0052] Erythorbic acid 1.25g
[0053] Water for injection 1000mL, adjust the pH to 6.7
[0054] Preparation:
[0055]Step 1: at 10°C, weigh the amount of tafluprost in the formula, add water for injection, stir to form an emulsion, add modified polylysine, and form nano micelles after stirring;
[0056] Step 2: Dissolving the stabilizer erythorbic acid in water for injection at 10°C to obtain an aqueous solution of the stabilizer erythorbic acid;
[0057] Step 3: At 10°C, mix the aqueous solution of the stabilizer erythorbic acid with the micelles obtained in Step 1, then add water for injection, and stir to dissolve;
[0058] Step 4: Adjust the pH to 6.7, add sterilized water for injection to 1000mL, filter and fill, and sterilize at 125°C to obtain the finished preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com