Application of Src inhibitor in preparation of medicine for preventing or treating uric acid nephropathy
A technology for uric acid nephropathy and inhibitors, which is applied in the field of preparing drugs for the prevention or treatment of uric acid nephropathy, Src inhibitors, can solve the problems of obvious toxic and side effects, renal failure, etc., achieve no toxic and side effects, inhibit renal fibrosis, and give The effect of simple and convenient medicine method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The construction of embodiment 1 uric acid nephropathy animal model
[0031] Take 24 male SPF grade SD rats, 8 weeks old, weighing 200-220g, after 7 days of adaptive feeding, they are randomly divided into 4 groups, 6 rats in each group.
[0032] (1) Preparation of rat uric acid nephropathy model
[0033] According to the ratio of rat body weight with adenine 0.1g / Kg, potassium oxonate 1.5g / Kg, mix the two with distilled water to make a suspension with a final concentration of 80g / L, and use 20ml / kg / d every Oral administration twice a day for 21 consecutive days induces a renal fibrosis model in rats with uric acid nephropathy. All animal experiments complied with the Chinese regulations on the management and use of laboratory animals.
[0034] (2) Experimental grouping
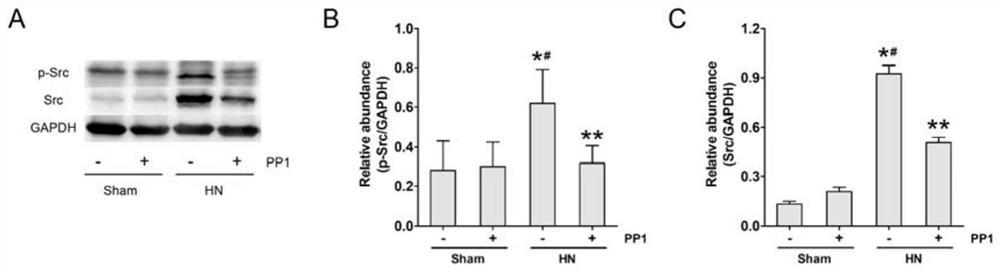
[0035] Inhibitor PP1 solution: Dissolve dimethyl sulfoxide (DMSO) in PBS to make 0.1% DMSO, then dissolve PP1 5mg / Kg in 50 μl of 0.1% DMSO (PP1 is an effective selective Src family inhibitor agent)...
Embodiment 2
[0041] Example 2 Retention of blood samples and detection of biochemical indicators
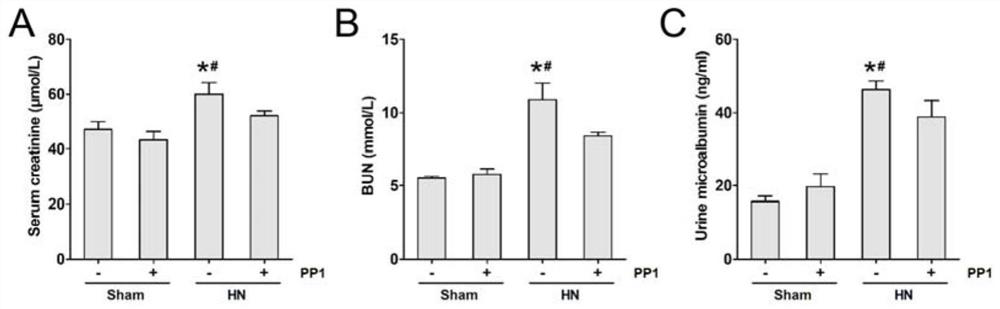
[0042] Fasting for 12 hours before blood collection, 2ml of blood was taken from the inner canthal vein or 5ml of blood was taken from the abdominal aorta after intraperitoneal injection of 3% pentobarbital sodium anesthetized according to the rat's body weight at a dose of 1ml / kg. Centrifuge at room temperature at 2500rpm for 15min, take the serum into a sterile Eppendorf tube, and freeze at -80°C for blood biochemical indicators to be tested. The blood samples and urine samples of each group of rats in Example 1 were measured by an automatic biochemical analyzer to measure serum creatinine (Serum creatinine, Scr), blood urea nitrogen (BUN) and urine microalbumin (Urine microalbumin). The result is as figure 1 shown.
[0043] figure 1 A shows: Compared with the sham group, the serum creatinine (Serum creatinine, Scr) level in the HN model group increased, and after the injection of PP1, t...
Embodiment 3
[0046] Example 3 Kidney Histomorphological Observation
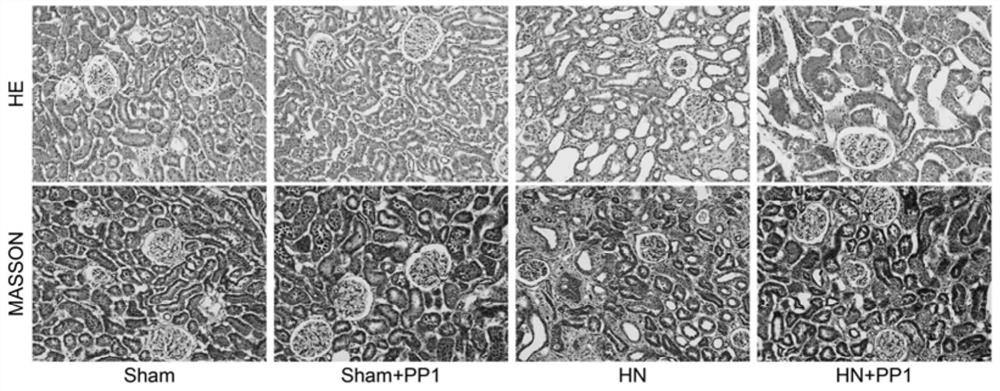
[0047] The kidney tissues of rats in each group obtained in Example 1 were taken and fixed with 4% paraformaldehyde. Routine graded ethanol dehydration, xylene transparent, paraffin embedded, 4μm serial paraffin sections, with HE and Masson staining to observe the pathological changes of renal tissue. The tubulointerstitial infiltration, renal thickness and fibrosis were observed under light microscope, and evaluated by Image-Pro-Plus software.
[0048] The result is as figure 2 Shown: HE staining results show that inflammatory cells infiltrate the interstitial area of the HN model, dilation of renal tubules, and the lesions are significantly relieved after PP1 treatment; Masson staining results show that compared with the sham group, the HN model shows the main components of fibrosis in the interstitial area of the kidney The blue-stained area of extracellular matrix such as collagen was significantly increased...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com