Monoterpenyl bishydroxycoumarin compound, pharmaceutical composition, preparation method of monoterpenyl bishydroxycoumarin compound, and application of monoterpenyl bishydroxycoumarin compound and pharmaceutical composition
A technology of dicoumarins and compounds, applied in the field of natural medicinal chemistry, can solve problems such as unclear etiology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0089] 1. Purpose of the experiment
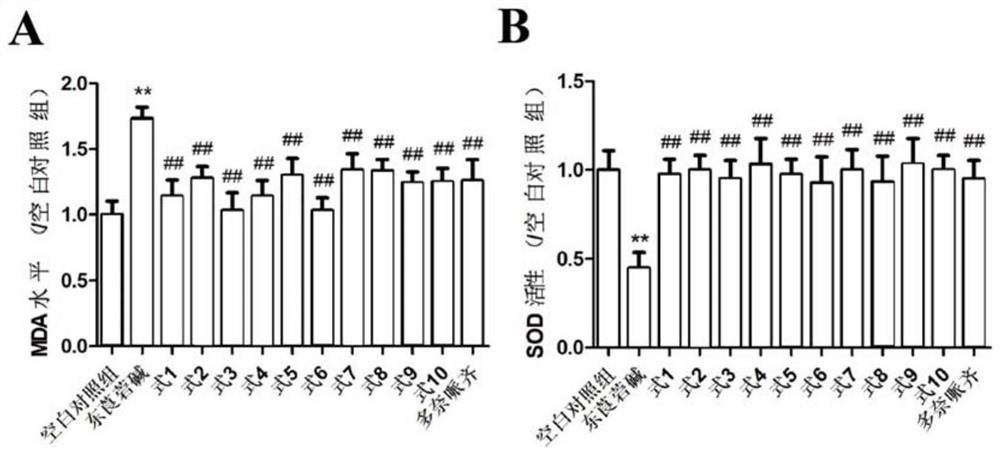
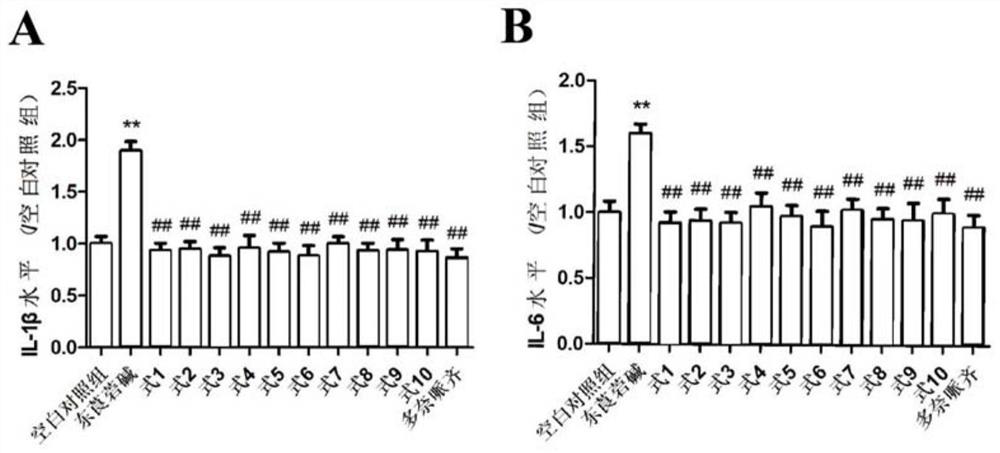
[0090] The SH-SY5Y cell model induced by the modeling agent scopolamine was used to study the neuroprotective effect of the compound, as well as the influence of oxidative stress and inflammation, and investigate the anti-senile dementia effect of compounds 1-10 of the present invention.
[0091] 2. Experimental method
[0092] 1. Experimental materials
[0093] Preparation of the tested sample solution: the tested sample is the pure compound 1-10 isolated and refined in the following example 1, and the compound 1-10 is precisely weighed and dissolved in DMSO, and the compound 1-10 is prepared to contain the concentration of the compound 1-10 respectively. The mother solution is 30mM, where mM is mmol / L, and the mother solution of each compound is diluted with DMSO to obtain the following concentration gradient solution, 0.1nM, 1nM, 10nM, 100nM, where nM is nmol / L.
[0094] Preparation of positive control solution (donepezil solution): a...
Embodiment 1
[0121] The preparation of embodiment 1 compound 1-10
[0122] (1) Take the bitter stem of Trigemina, add 12 times of the weight of the stem of Trigemina, and add 70% ethanol aqueous solution for reflux extraction once, heat reflux time at 92°C for 2h, collect the ethanol extract; The volume ratio of 10 times the weight of the root is 70% ethanol aqueous solution for reflux extraction once, heat reflux time at 92 ° C for 2 hours, and collect the ethanol extract; the remaining medicinal residues are added to the volume ratio of 8 times the weight of the root of Trident bitter root to be 70% The ethanol aqueous solution was refluxed and extracted once, and the reflux time was 2 hours, and the ethanol extract was collected; the ethanol extracts were combined, concentrated under reduced pressure until no alcohol smell, and 4 kg of ethanol extract was obtained.
[0123] (2) After dissolving 4 kg of ethanol extract with distilled water, extract with sherwood oil, ethyl acetate, n-but...
Embodiment 2
[0127] The characterization of embodiment 2 racemate 1-5 and compound 1-10
[0128]
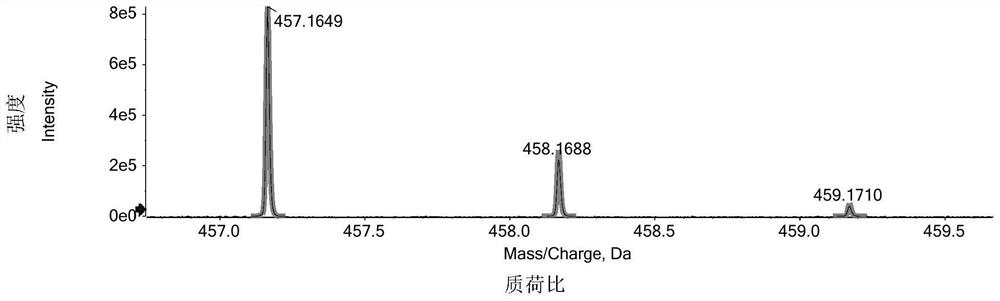
[0129] Racemate 1 is a white amorphous powder, and the HR-ESI-MS spectrum gives a quasi-molecular ion peak m / z 457.1649[M+H] + (calcd.for 457.1651), combined with 13 C NMR spectrum deduces that the molecular formula of compound 1 and compound 2 is C 28 h 24 o 6 . Ultraviolet spectrum (UV) shows characteristic absorption of coumarin (λ max 202nm, 246nm, 333nm); Infrared spectrum (IR) showed hydroxyl absorption (3647cm -1 ), carbonyl absorption (1716cm -1 ).
[0130] 1 In the H-NMR spectrum, a set of characteristic hydrogen signals of coumarin nuclei were observed in the low field region: δ H 6.10 (1H, d, J = 9.4Hz, H-3) and δ H 7.79 (1H, d, J = 9.4Hz, H-4); a set of E-configuration double bond proton signals: δ H 5.96(1H,d,J=16.4Hz,H-9') and δ H 6.71 (1H, d, J=16.4Hz, H-10′); in addition, there are two ene hydrogen proton signals in the low field region: δ H 5.30(1H,brs,H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com