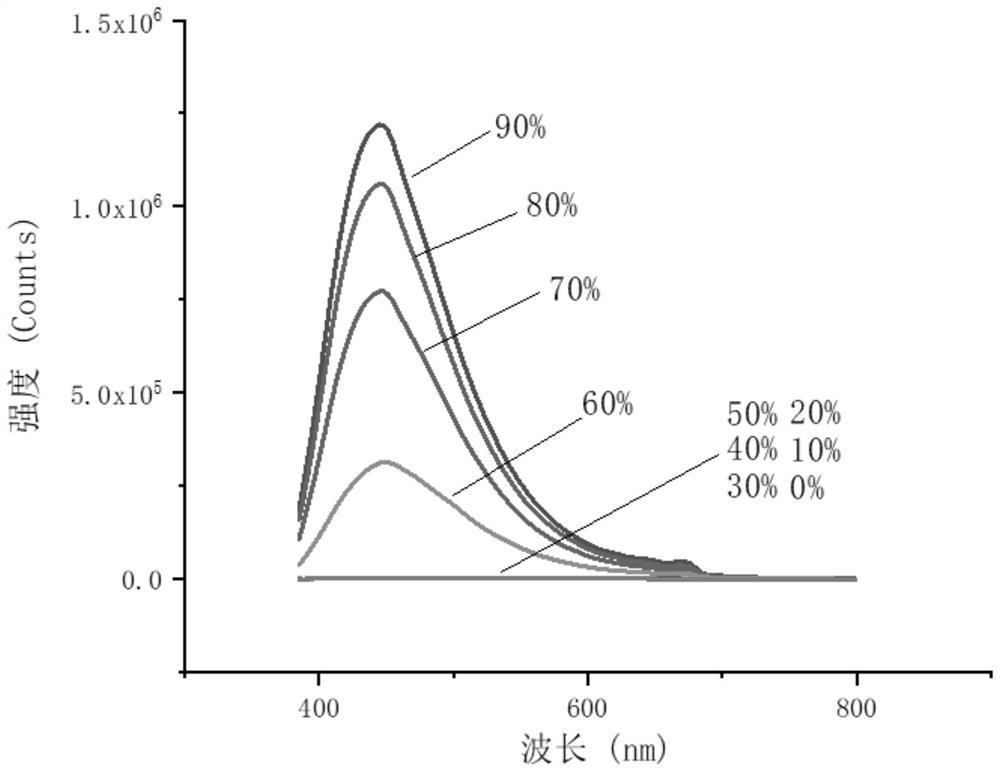
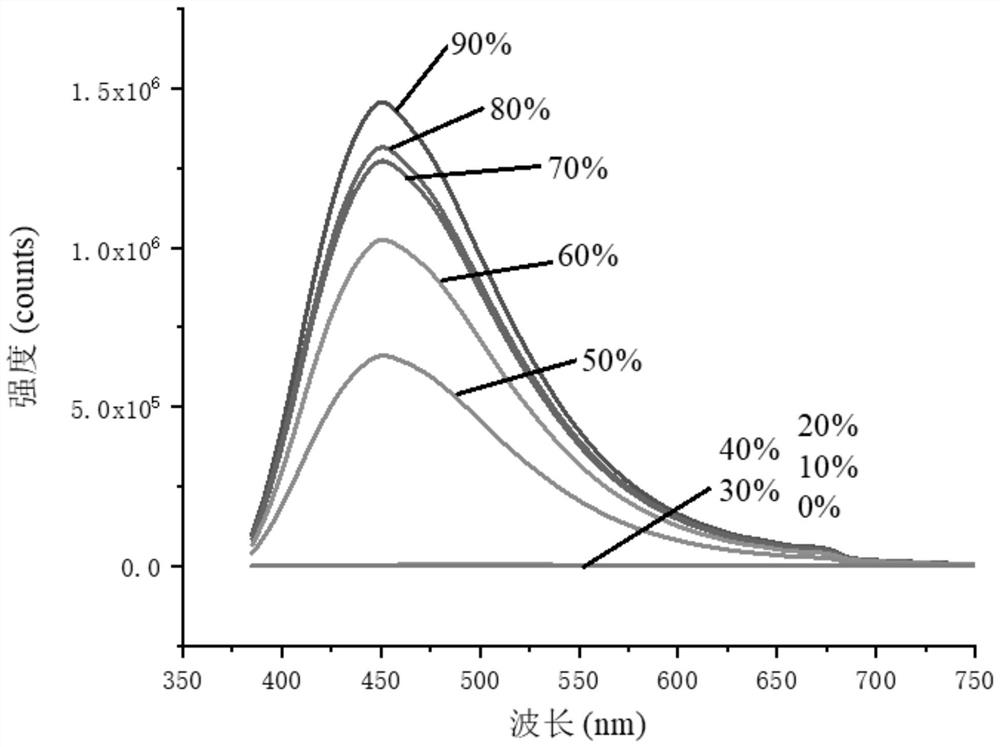
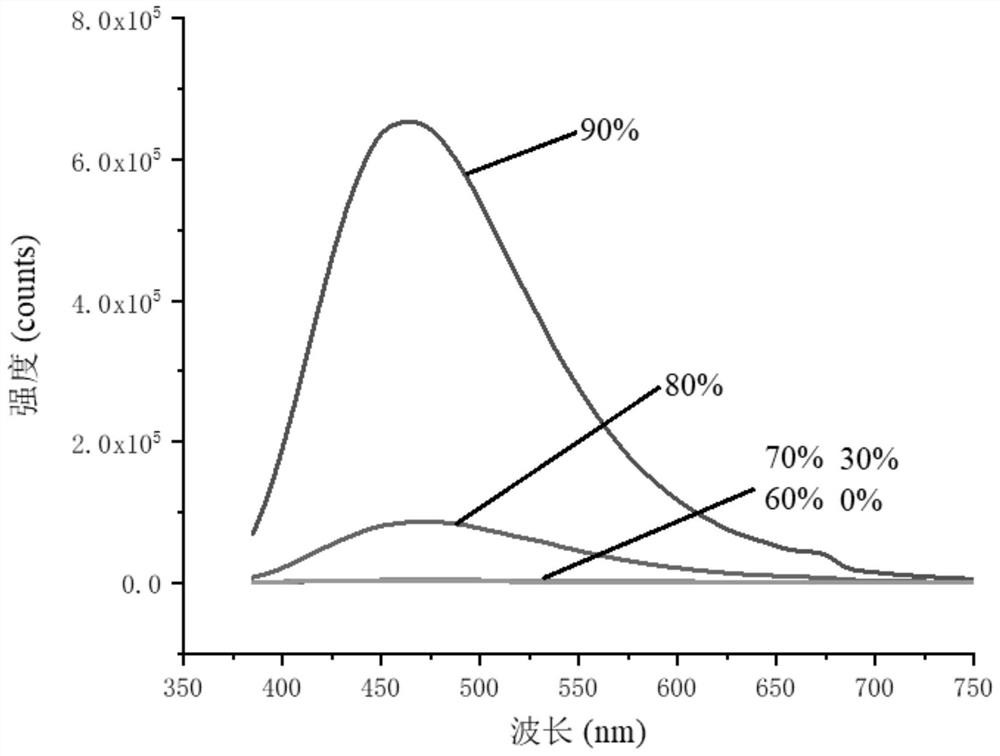
4-Phenyl-6h-1,3-oxazin-6-one derivatives and their preparation and application
A derivative, phenyl technology, applied in 4-phenyl-6H-1,3-oxazin-6-one derivatives and their preparation and application fields, can solve the problems of cumbersome post-processing, unfriendly environment, bottom It can solve the problems of large limitations and other problems, and achieve the effects of wide substrate universality, high atom utilization, simple and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Synthesis of 2-(tert-butylamino)-5-methyl-4-phenyl-6H-1,3-oxazin-6-one
[0052] Weigh 0.2mmol of 4-methyl-3-phenylisoxazol-5(4H)-one (the compound corresponding to No. (1), 0.0350g), 0.02mmol of palladium dichloride (0.0036g), 0.04mmol of sodium benzoate (0.0058g), in a 10mL test tube reaction tube, add 2mL 1,1,2-trichloroethane as a solvent, and finally add tert-butylisonitrile (the compound corresponding to No. (17), 45μL) with a rubber stopper for the test tube mouth Sealed, stirred and reacted at 85 ° C for 6 hours; after the reaction was completed, the reaction solution was successively subjected to vacuum concentration and column chromatography separation (column chromatography separation conditions: the stationary phase was 200~300 mesh silica gel powder, and the mobile phase was ethyl acetate (A ) and petroleum ether (B), the mobile phase change program (A:B) was 1:10) to obtain 0.0438 g of the reaction product.
[0053] The above reaction products were charact...
Embodiment 2
[0056] Synthesis of 2-(tert-butylamino)-5-methyl-4-(p-tolyl)-6H-1,3-oxazin-6-one
[0057] 4-Methyl-3-p-tolylisoxazol-5(4H)-one (the compound corresponding to No. (2), 0.0378g), 0.02mmol palladium dichloride (0.0036g), 0.04mmol sodium benzoate (0.0058g) g), in a 10 mL test tube reaction tube, add 2 mL of 1,1,2-trichloroethane as a solvent, and finally add tert-butyl isonitrile (the compound corresponding to No. (17), 45 microliters), and seal the mouth of the test tube with a rubber stopper , the reaction was stirred at 85°C for 6 hours; after the reaction, the reaction solution was successively subjected to vacuum concentration and column chromatography separation (column chromatography separation conditions: the stationary phase was 200-300 mesh silica gel powder, and the mobile phase was ethyl acetate (A) and petroleum ether (B), the mobile phase change program (A:B) was 1:10) to obtain 0.0533 g of the reaction product.
[0058] The above reaction products were characterize...
Embodiment 3
[0061] Synthesis of 2-(tert-butylamino)-5-methyl-4-(p-methoxyphenyl)-6H-1,3-oxazin-6-one
[0062] 3-(4-Methoxyphenyl)-4-methyl-isoxazol-5(4H)-one (the compound corresponding to No. (3), 0.0410 g), 0.02 mmol palladium dichloride (0.0036 g) , 0.04mmol sodium benzoate (0.0058g), in a 10mL test tube reaction tube, add 2mL 1,1,2-trichloroethane as a solvent, and finally add the compound corresponding to tert-butylisonitrile (No. (17), 45 μL ) The mouth of the test tube was closed with a rubber stopper, and the reaction was stirred at 85°C for 6 hours; after the reaction was completed, the reaction solution was successively subjected to vacuum concentration and column chromatography separation (column chromatography separation conditions: the stationary phase was 200-300 mesh silica gel powder, and the mobile phase was It was ethyl acetate (A) and petroleum ether (B), and the mobile phase change program (A:B) was 1:10) to obtain 0.0484 g of the reaction product.
[0063] The above ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com