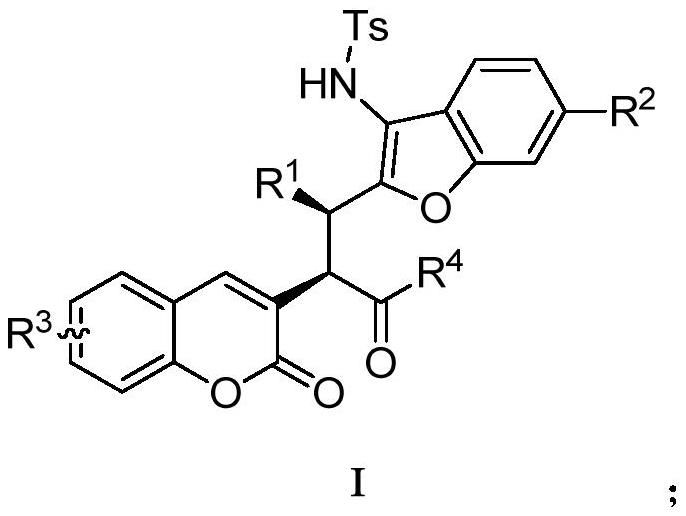
A kind of preparation method of benzofuranocoumarin compound
A technology of benzofuran and compounds, applied in the field of organic chemical drug synthesis, to achieve the effect of large industrialization potential, low price, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
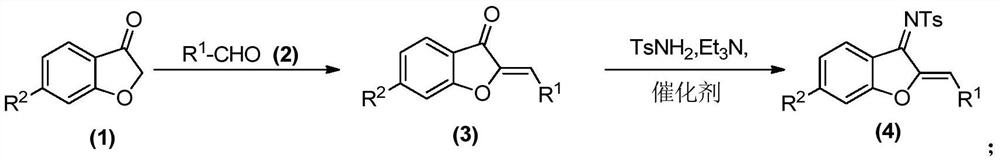
[0052] The preparation of compound 001 to compound 022 was carried out with reference to the above-mentioned synthetic route 1.
[0053] 1. The specific route of compound 001 is shown in the following formula:
[0054]
[0055] Concrete preparation process is as follows:
[0056] Step S1:
[0057] (1) In a round bottom flask, benzofuran (1.0 eq) of formula (1) was added and dissolved in dichloromethane (50.0 mL). After the round bottom flask was stirred at room temperature for 5 min, aluminum oxide (36.0 eq) and benzaldehyde (1.05 eq) were added in sequence, and the reaction was stirred at room temperature for 3 hours. After the reaction, the alumina was removed by suction filtration, the filtrate was collected, concentrated and dried to obtain a crude product, which was recrystallized or separated by a silica gel column to obtain the compound of formula (3).
[0058] (2) In a round bottom flask, dissolve the compound of formula (3) (1.0eq) and p-toluenesulfonamide (1.5e...
Embodiment 2
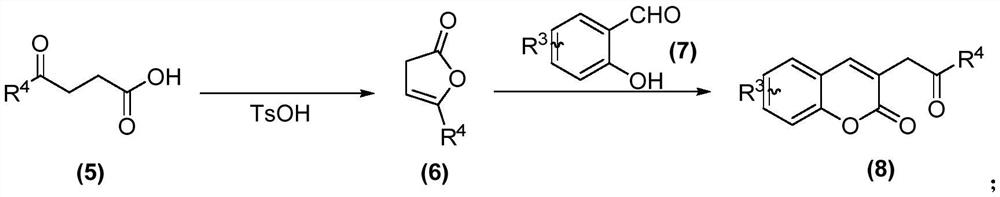
[0154] The preparation of compound 023 to compound 029 was carried out with reference to the above-mentioned synthetic route 2.
[0155]1. The specific preparation process of compound 023 is as follows:
[0156] Step S1:
[0157] (1) In a round bottom flask, benzofuran (1.0 eq) of formula (1) was added and dissolved in dichloromethane (50.0 mL). After the round bottom flask was stirred at room temperature for 5 min, aluminum oxide (36.0 eq) and benzaldehyde (1.05 eq) were added in sequence, and the reaction was stirred at room temperature for 3 hours. After the reaction, the alumina was removed by suction filtration, the filtrate was collected, concentrated and dried to obtain a crude product, which was recrystallized or separated by a silica gel column to obtain the compound of formula (3).
[0158] (2) In a round bottom flask, dissolve the compound of formula (3) (1.0eq) and p-toluenesulfonamide (1.5eq) in toluene (40mL), and add triethylamine in sequence at 0°C under nitr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com