Application of huGEMM PD-1 mouse to evaluation of drug effects and immune-related side effects of in-vivo humanized PD-1 antibody
A PD-1 and immune-related technology, applied in the field of biomedicine, can solve the problems of immune cell function impact, mouse weight loss, and low tumor heterogeneity, so as to improve the degree of immune reconstruction, reduce GVHD, and have good application prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
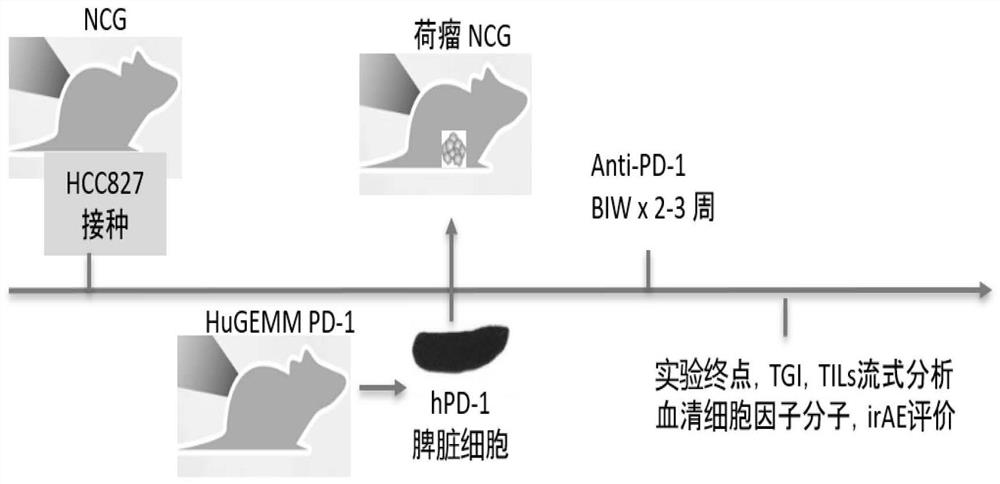
[0037] This embodiment includes the following main steps, as attached figure 1 Shown:
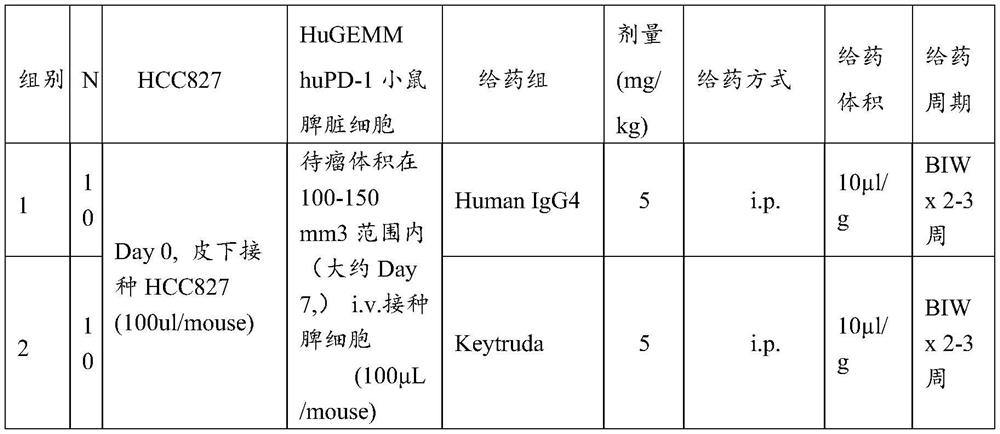
[0038] 1. Inoculate human non-small cell lung cancer cells in immunodeficient mice to establish a mouse model of human non-small cell lung cancer;
[0039] 2. After successful modeling, use huGEMM hPD-1 mice ((B6 / JGpt-Pdcd1 em1Cin(hPDCD1) / Gpt, Cat#T003095, Jicui Yaokang)-derived spleen cells, adoptively infused into immunodeficient mice in step 1 for immune reconstitution,
[0040] 3. Immunodeficient mice after immune reconstitution are used to evaluate the efficacy and immune-related side effects of human-derived PD-1 / PD-L1 antibody drugs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com