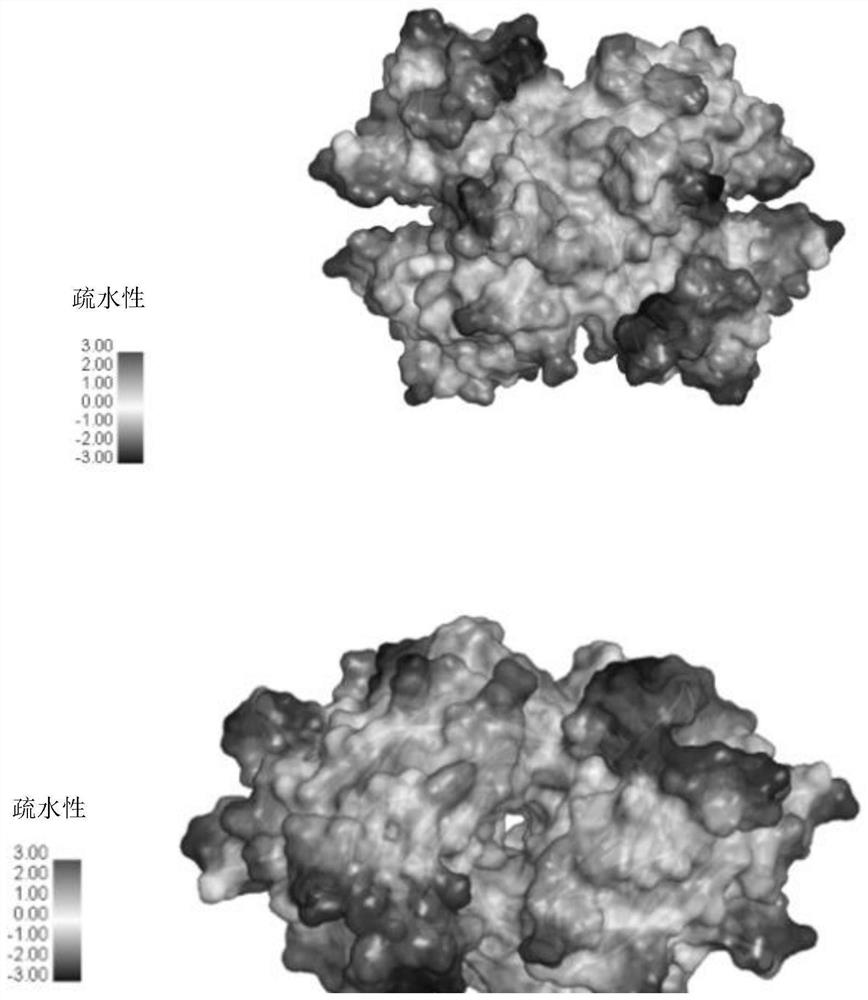
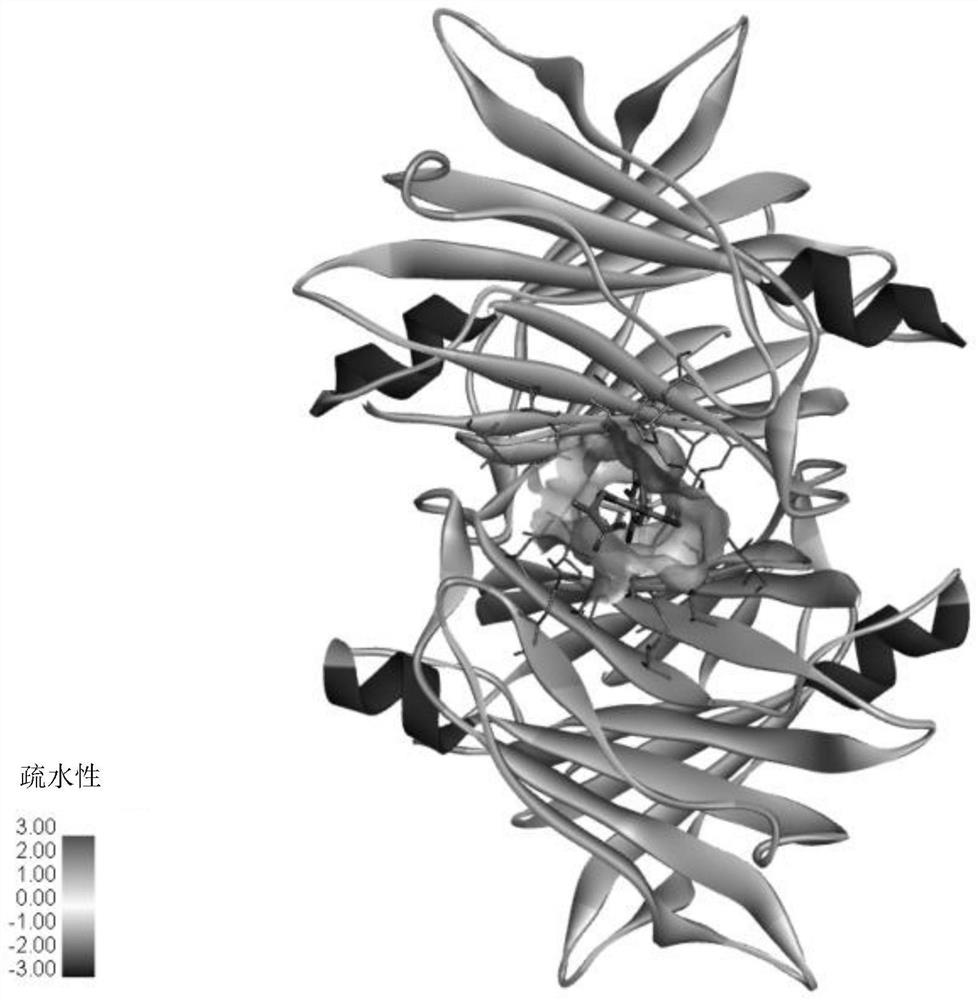
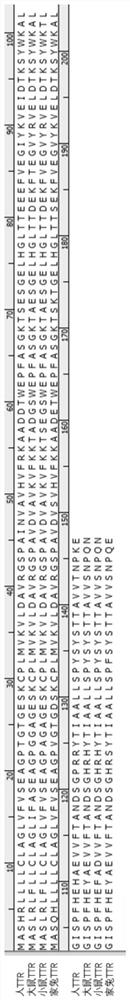
Ophthalmic preparation as well as preparation method and application thereof
An ophthalmic preparation and ocular technology, applied in the field of protein engineering, can solve problems such as poor therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Embodiment 1 Recombinant preparation of human transthyretin (TTR)
[0072] (1) Construction of the recombinant plasmid pETx-rhaPBAD-ttr: transform and restructure the pET-21a plasmid (purchased from the ATCC China Culture Collection Center) (the difference in sequence between the restructured plasmid and the original pET-21a plasmid is About 75%, the specific sequence is shown in SEQ ID NO: 4), and the rhaPBAD promoter (Rhamna Tang inducible) is used to replace the T7 promoter, and the human TTR optimized nucleic acid sequence is connected at the same time (as shown in SEQ ID NO: 2 , the amino acid sequence of TTR is shown in SEQ ID NO:1), and the overall nucleic acid sequence of the resulting plasmid is shown in SEQ ID NO:3. After sequencing verification (the sequencing company is Nanjing GenScript Biotechnology Co., Ltd.), the construction was successful.
[0073] (2) Expression and purification of recombinant human TTR: transform the pETx-rhaPBAD-ttr plasmid constru...
Embodiment 2
[0076] Example 2 Using sodium carboxymethylcellulose as an auxiliary material can promote TTR to pass through the corneal barrier
[0077] (1) Configure the human TTR prepared in Example 1 to be 5-30 μmol / L (containing physiological saline), and apply eye drops to C57BL / 6 mice (8 weeks old) and SD rats (8 weeks old) respectively After 3 hours, they were killed, and the vitreous and fundus samples were taken to extract protein. Using rabbit anti-His-tag antibody as the primary antibody and donkey anti-rabbit antibody as the secondary antibody, the vitreous and SD rats were measured by ELISA. Content of human TTR in fundus samples.
[0078] (2) The human-derived TTR prepared in Example 1 was configured as 10 μmol / L (containing physiological saline and 0-8 mg / mL sodium carboxymethylcellulose (purchased from Sinopharm, with a viscosity of 800-1200CP)), respectively for C57BL / 6 mice (8 weeks old) and SD rats (8 weeks old) were given eye drops, and sacrificed after 3-72 hours, and...
Embodiment 3
[0082] Example 3 Using dextran 70 as an auxiliary material can promote TTR to pass through the corneal barrier
[0083] (1) Configure the human TTR prepared in Example 1 to be 5-30 μmol / L (containing physiological saline), and apply eye drops to C57BL / 6 mice (8 weeks old) and SD rats (8 weeks old) respectively After 3 hours, they were killed, and the vitreous and fundus samples were taken to extract protein. Using rabbit anti-His-tag antibody as the primary antibody and donkey anti-rabbit antibody as the secondary antibody, the vitreous and SD rats were measured by ELISA. Content of human TTR in fundus samples.
[0084] (2) The human TTR prepared in Example 1 was configured to be 10 μmol / L (containing normal saline and 0-0.8 mg / mL dextran 70 (purchased from Sinopharm, with a molecular weight of 64000-76000)), respectively for C57BL / 6 small Rats (8 weeks old) and SD rats (8 weeks old) were given eye drops, and sacrificed after 3-72 hours, and the vitreous and fundus samples we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com