Method for synergistically catalyzing oxidization of cycloalkane by using metalloporphyrin MOFs PCN-224(Mn)/Zn (II) salt
A mofspcn-224, metalloporphyrin technology is applied in the field of industrial catalysis and fine organic synthesis, and can solve the problems of reducing the selectivity of cycloalkyl alcohols and cycloalkyl ketones, increasing the uncontrollability of the reaction system, poor selectivity of target products, and the like, Achieve high selectivity, inhibition of disordered diffusion, and small environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
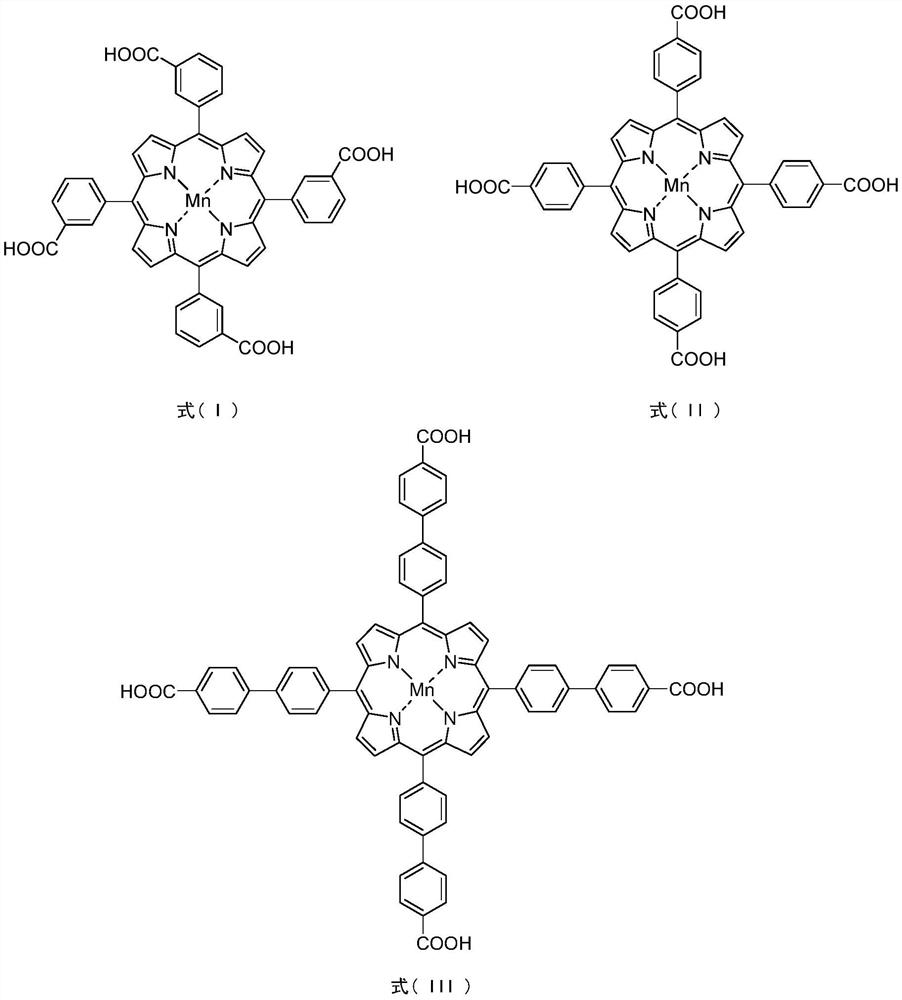
[0028] Synthesis of PCN-224(Mn)-m: In a 35mL pressure-resistant reaction tube, T(3-COOH)PPMn(II) (0.0847g, 0.1mmol), ZrCl 4 (0.1400g, 0.6mmol), benzoic acid (5.4000g, 44.3mmol) was dissolved in 16.0mL DMF, ultrasonic 30min until completely dissolved. The mixture was placed in an electric constant temperature blast drying oven at 120° C. for 48.0 hours of static reaction. After the reaction is complete, turn off the heating, cool naturally to room temperature, filter the crude product with suction and rinse with DMF and acetone successively, then transfer to a 10.0 mL centrifuge tube, centrifuge for 5.0 min (3000 rpm) in a low-speed centrifuge, pour out the upper layer, and dry DMF leaching (3×8.0mL) until the upper layer is clear, dry acetone leaching (3×8.0mL) until the upper layer is clear, remove the solid in the lower layer, and dry at 90°C for 8.0h to obtain a brick red powder (0.0680g, 44.7% yield).
Embodiment 2
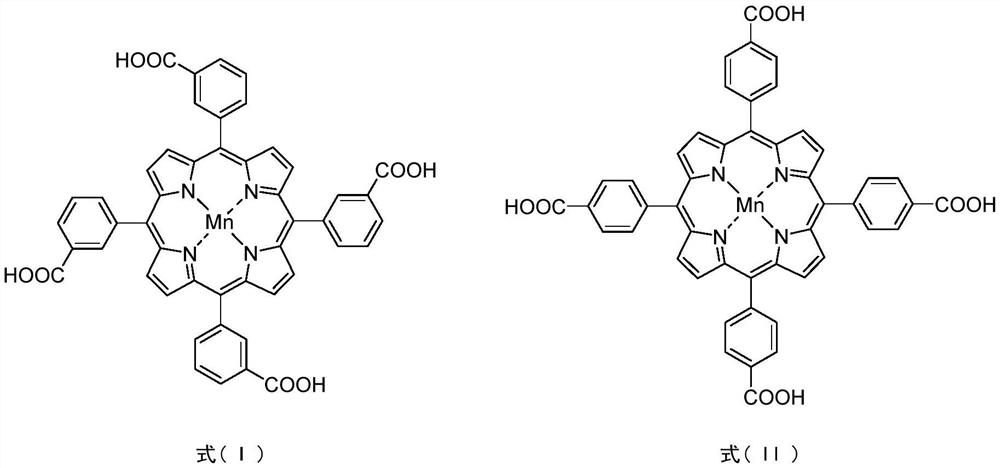
[0030] Synthesis of PCN-224(Mn)-p: In a 35mL pressure-resistant reaction tube, T(4-COOH)PPMn(II) (0.0847g, 0.1mmol), ZrCl 4(0.1400g, 0.6mmol), benzoic acid (5.4000g, 44.3mmol) was dissolved in 16.0mL DMF, ultrasonic 30min until completely dissolved. The mixture was placed in an electric constant temperature blast drying oven at 120° C. for 48.0 hours of static reaction. After the reaction is complete, turn off the heating, cool naturally to room temperature, filter the crude product with suction and rinse with DMF and acetone successively, then transfer to a 10.0 mL centrifuge tube, centrifuge for 5.0 min (3000 rpm) in a low-speed centrifuge, pour out the upper layer, and dry DMF leaching (3×8.0mL) until the upper layer is clear, dry acetone leaching (3×8.0mL) until the upper layer is clear, remove the solid in the lower layer, and dry at 90°C for 8.0h to obtain a brick red powder (0.0690g, 45.3% yield).
Embodiment 3
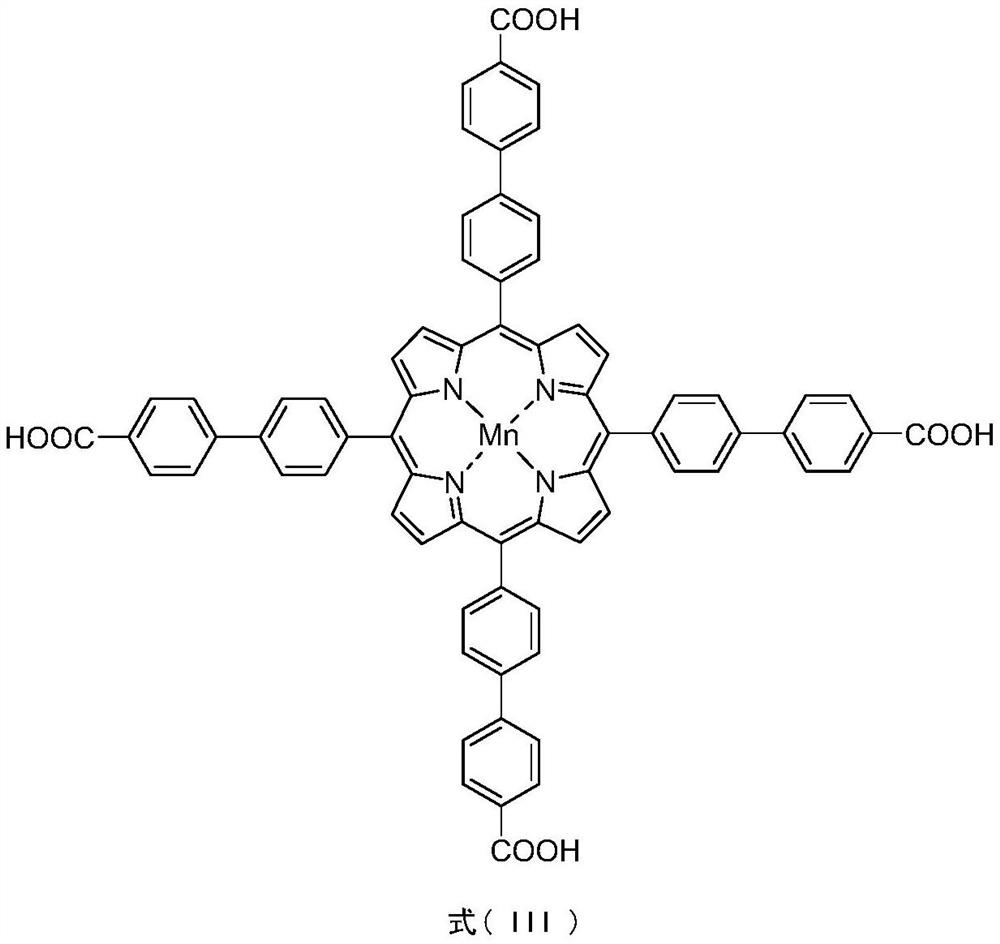
[0032] Synthesis of PCN-224(Mn)-d: In a 35mL pressure-resistant reaction tube, [T(4-(4-COOH)P)PPMn(II)](0.1152g, 0.1mmol), ZrCl 4 (0.1400g, 0.6mmol), benzoic acid (5.4000g, 44.3mmol) was dissolved in 16.0mL DMF, ultrasonic 30min until completely dissolved. The mixture was placed in an electric constant temperature blast drying oven at 120° C. for 48.0 hours of static reaction. After the reaction is completed, turn off the heating, cool to room temperature naturally, filter the crude product with suction and rinse with DMF and acetone successively, then transfer to a 10.0mL centrifuge tube, centrifuge at 5.0m (3000.0rpm) in a low-speed centrifuge, pour out the upper layer liquid, Dry DMF leaching (3×8.0mL) until the upper layer is clear, dry acetone leaching (3×8.0mL) until the upper layer is clear, remove the solid in the lower layer, and dry at 90°C for 8.0h to obtain a brick red powder (0.0670g, 43.3% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com