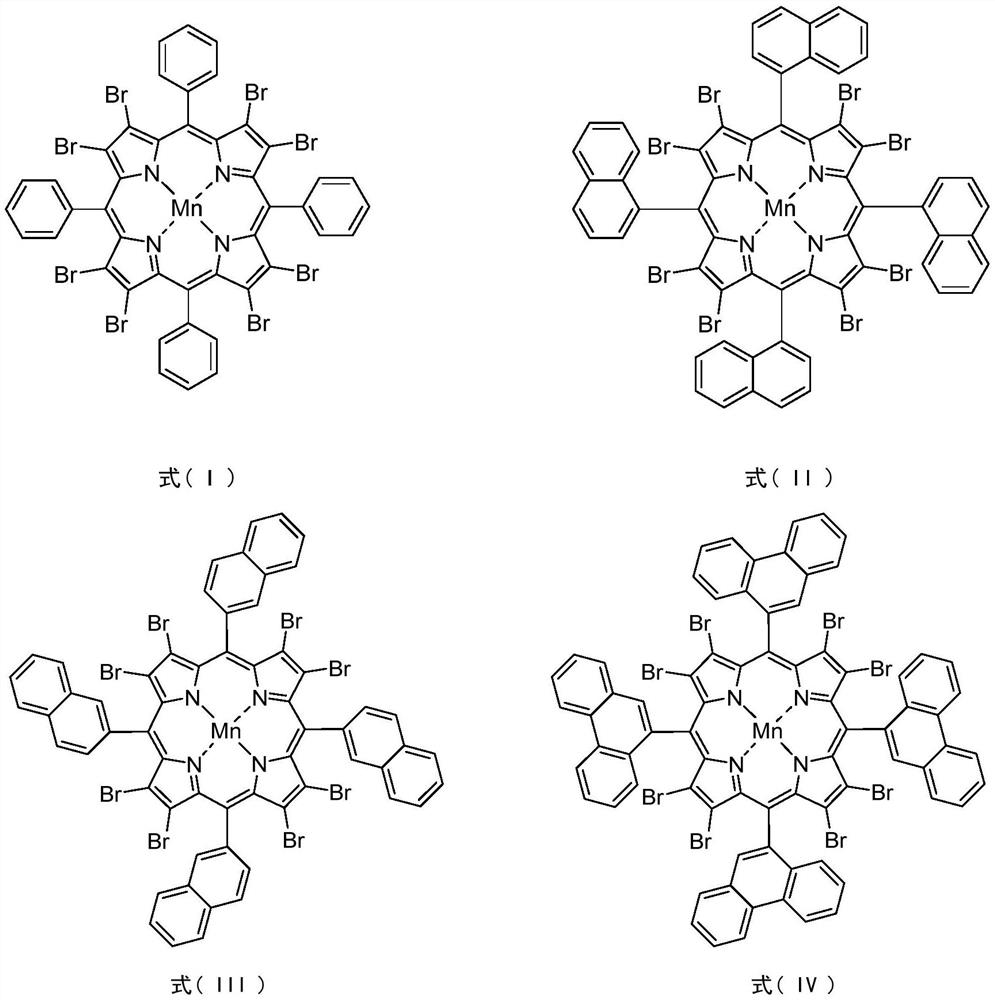
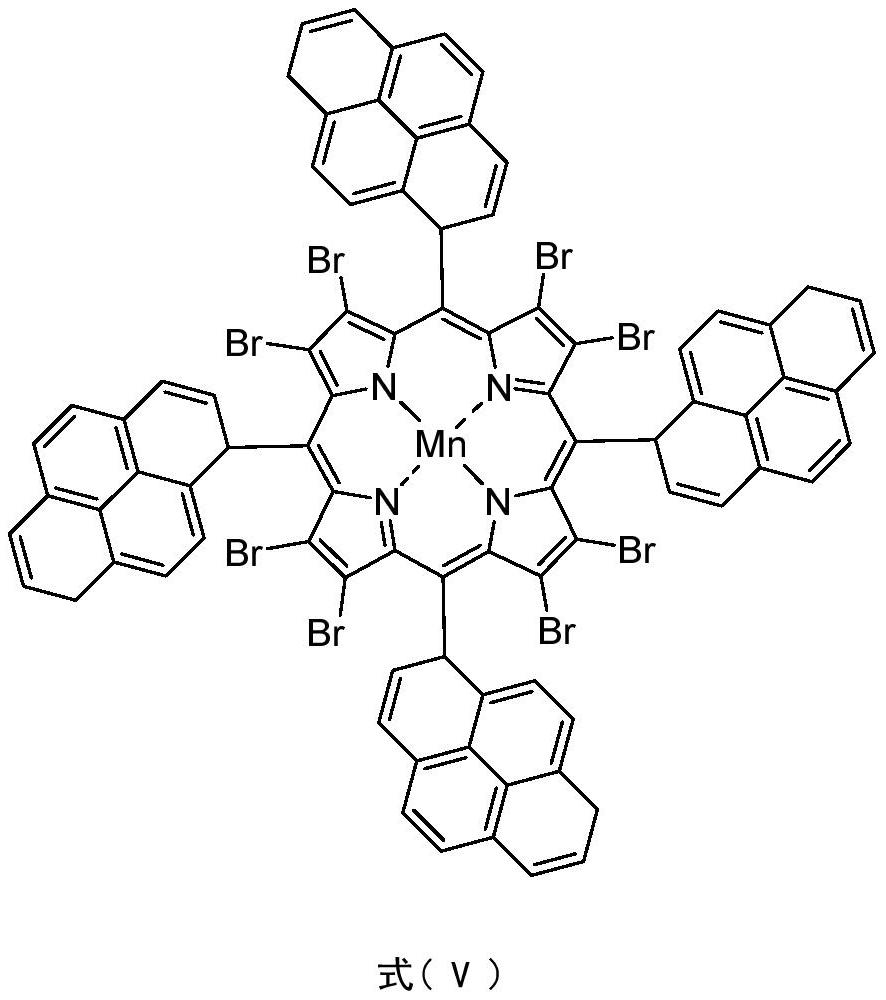
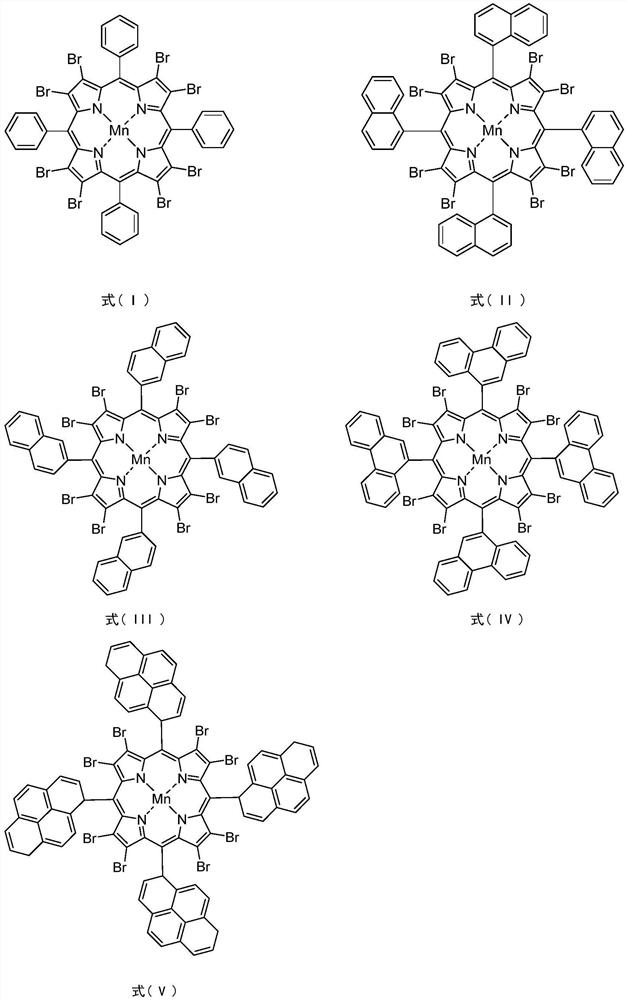
Method for synergistically catalyzing and oxidizing cycloparaffin through confined metalloporphyrin manganese (II)/Zn (II) salt
A technology of synergistic catalysis and manganese porphyrin, applied in chemical instruments and methods, physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, etc., can solve the problem of reducing the selection of cycloalkyl alcohols and cycloalkyl ketones Problems such as non-resistance, increasing the uncontrollability of the reaction system, and poor selectivity of the target product can achieve the effects of high selectivity, inhibition of disordered diffusion, and low environmental impact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 47
[0024] Example 47 is a scale-up experiment of catalytic oxidation of naphthenes.
Embodiment 1
[0026] In a 100mL stainless steel autoclave with polytetrafluoroethylene liner, 0.0036g (0.0024mmol) 5,10,15,20-tetrakis(2-naphthyl)-2,3,7,8,12,13 , 17,18-octabromoporphyrin manganese (II) and 0.4036g (2.200mmol) zinc acetate are dispersed in 16.8320g (200mmol) cyclohexane, seal the reaction vessel, stir and heat up to 120°C, and feed oxygen to 1.00MPa . Stir and react at 120°C, 1.0MPa oxygen pressure, 600rpm for 8.0h. After completion of the reaction, cool to room temperature with ice water, add 1.3115g (5.00mmol) triphenylphosphine (PPh 3), stirred at room temperature for 30min to reduce the generated peroxide. Using acetone as solvent, the resulting reaction mixture was adjusted to 100 mL. Pipette 10 mL of the resulting solution, and use toluene as an internal standard for gas chromatography analysis; pipette 10 mL of the resulting solution, and use benzoic acid as an internal standard for liquid chromatography analysis. The conversion rate of cyclohexane was 7.06%, the...
Embodiment 2
[0028] In a 100mL stainless steel autoclave with polytetrafluoroethylene liner, 0.0036g (0.0024mmol) 5,10,15,20-tetrakis(1-naphthyl)-2,3,7,8,12,13 ,17,18-Octabromoporphyrin manganese (II) and 0.4036g (2.200mmol) zinc acetate are dispersed in 16.8320g (200mmol) cyclohexane, seal the reaction vessel, stir and heat up to 120°C, and feed oxygen to 1.00MPa . Stir and react at 120°C, 1.0MPa oxygen pressure, 600rpm for 8.0h. After completion of the reaction, cool to room temperature with ice water, add 1.3115g (5.00mmol) triphenylphosphine (PPh 3 ), stirred at room temperature for 30min to reduce the generated peroxide. Using acetone as solvent, the resulting reaction mixture was adjusted to 100 mL. Pipette 10 mL of the resulting solution, and use toluene as an internal standard for gas chromatography analysis; pipette 10 mL of the resulting solution, and use benzoic acid as an internal standard for liquid chromatography analysis. The conversion rate of cyclohexane is 6.88%, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com