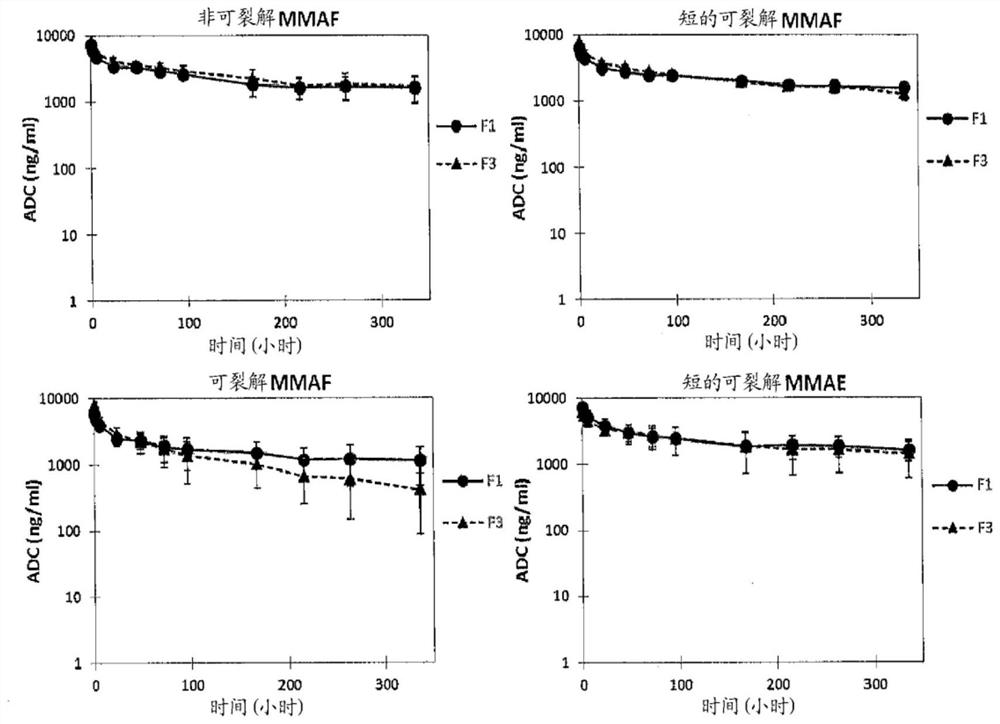
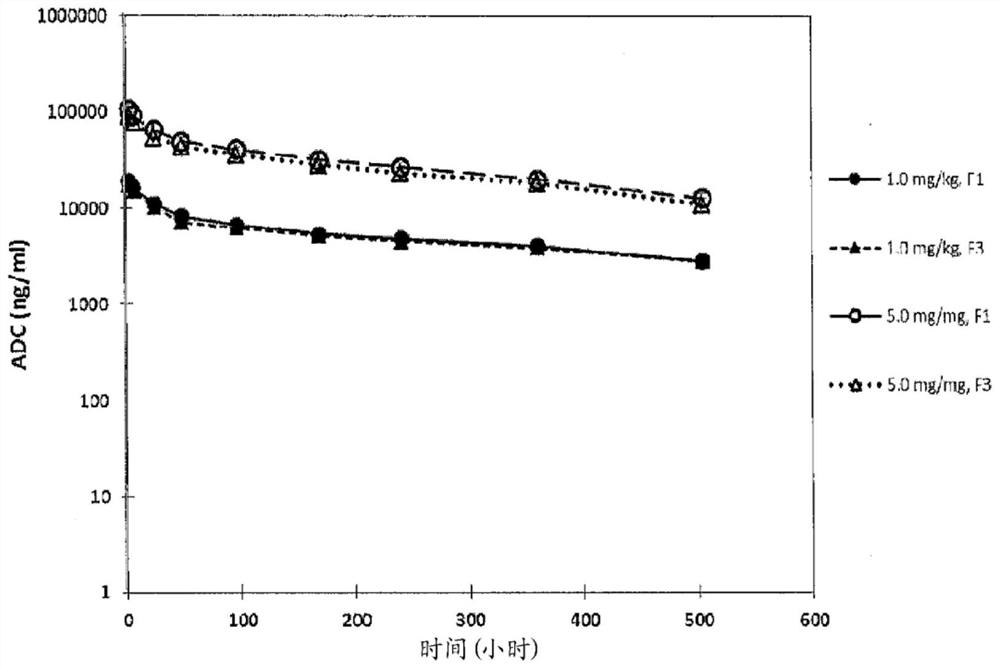
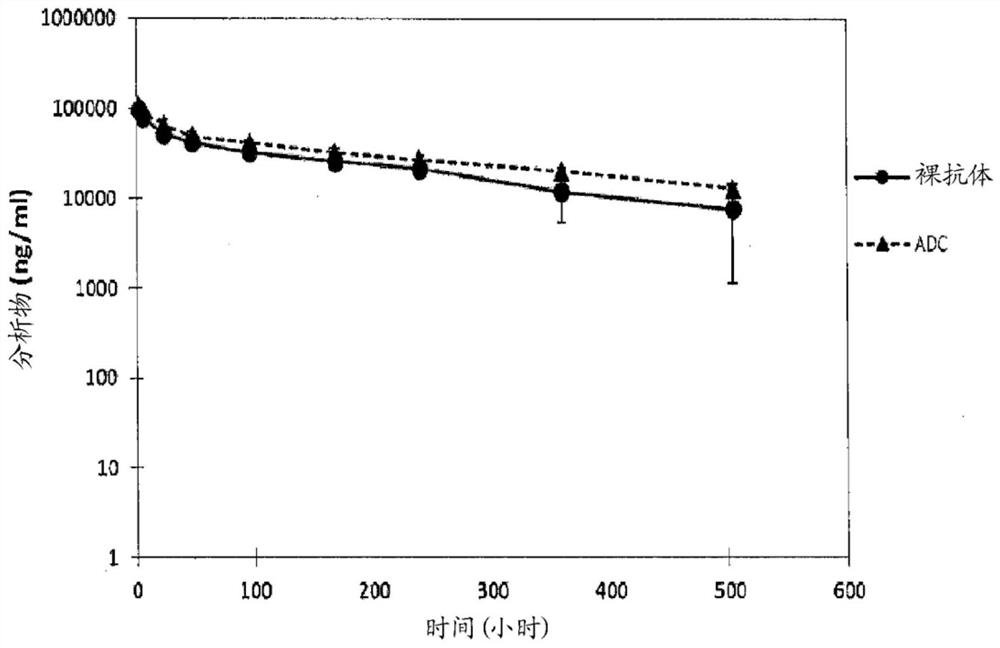
Humanized Anti-prostate-specific membrane antigen (PSMA) antibody drug conjugates
A prostate-specific, antibody drug technology, applied in a method and composition for the prevention or treatment of PSMA-related diseases or cancer, in the field of inhibition, which can solve problems such as lack of therapeutic index and toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0182] Example 1: Transient transfection. Approximately 16 hours before transfection, the CHO-S culture was incubated at 0.75x10 6 / mL in FreeStyle CHO medium. The next day, when the cell count reaches 1.4–1.6x10 6 / mL, the cells were transfected. At target cell counts, 400 mM p-acetylphenylalanine (pAF) stock was added to achieve a final culture concentration of 1.4 mM.
[0183] Polyethyleneimine / DNA (PEI / DNA) complexes were prepared as described: DNA (1.42ug / 1x10 6 cells) were dissolved in RPMI medium (5% (v / v) of the total culture volume), the DNA / RPMI mixture was incubated at room temperature for 2 minutes, and the PEI stock (1 mg / mL) was dissolved in a 3:1 ratio ( PEI / DNA (ug / ug)) was added to the DNA solution, and the mixture was incubated at room temperature for 5 minutes.
[0184] The mixture was added gently to the cell culture and vortexed. Transfer the flask to a 32 °C incubator. On day 6 after transfection, western blot analysis was performed. On day 7 afte...
Embodiment 2
[0185] Example 2: Antibody humanization - parental mouse J591 (Liu et al., Cancer Research, 57, 3629-36354, 1997) antibody was humanized by selecting a human framework based on sequence identity to the mouse framework sequence. Light and heavy chain CDRs from mouse antibodies were grafted onto human frameworks separately and analyzed for binding to PSMA antigen. Humanized variants were generated by pairing four human heavy chain frameworks with six light chain frameworks. The variants were transiently expressed in HEK293 cells and supernatants were tested by FACS for binding to PSMA antigen expressed in LnCap cells. Table 4 describes selected heavy chain variable region sequences and 4 light chain variable region sequences used for further studies. Binding assays revealed that four humanized full-length variants, shown as humanized anti-PSMA variants 1, 2, 3 and 4 (Table 4), retained similar binding affinities to the chimeras (Table 4), exhibiting nanovolume Molar concentrat...
Embodiment 3
[0191]Example 3: Recombinant Expression of Anti-PSMA Antibodies - Lead humanized anti-PSMA antibodies were selected and expressed recombinantly in CHO cells by both transient transfection (described in Example 1) and stable mass confluency methods to examine human Expression of the sourced sequence. In the transient transfection and stable mass confluency approaches, proprietary technologies are built into expression vectors and CHO host cells, respectively (see eg WO2018 / 223108). The unnatural amino acid p-acetylphenylalanine (pAF) was incorporated in the PSMA antibody at position A114 (Kabat numbering scheme, (see Kabat et al., NIH Publication No. 369-847, 1993); the position is given in Table 4 by A indicated), said position being the first amino acid residue of the constant region of the heavy chain.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com