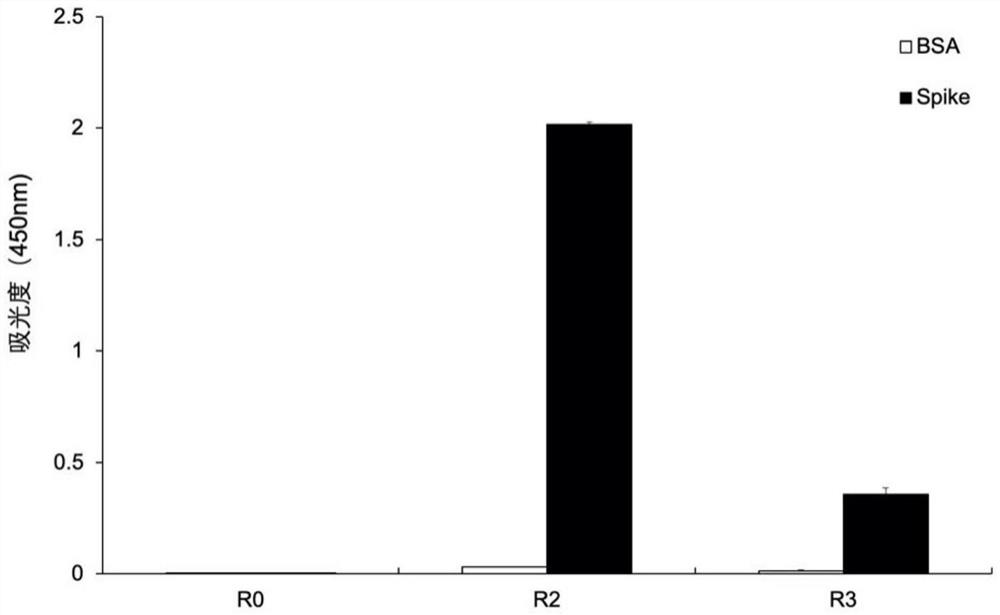
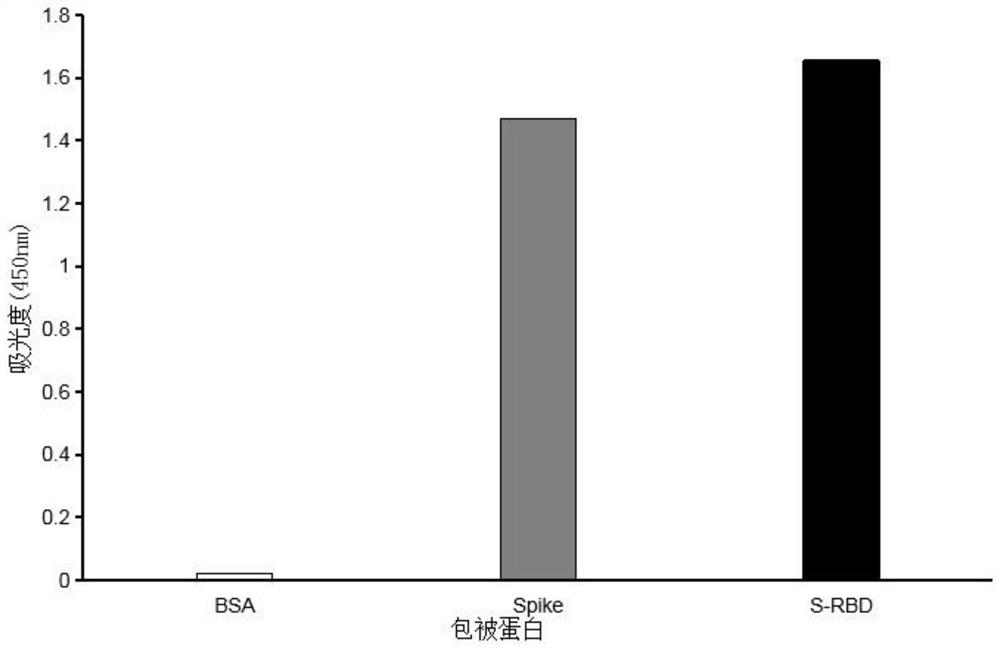
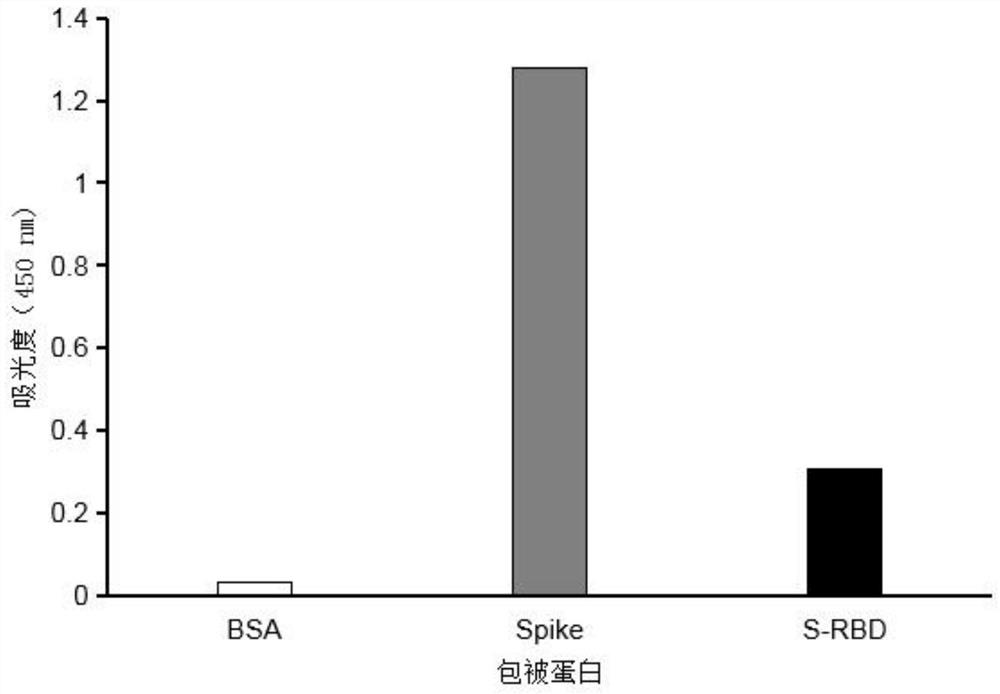
Monoclonal antibody for novel coronavirus virus SARS-CoV-2 spike protein RBD region and application of monoclonal antibody
A monoclonal antibody and spike protein technology, applied in the fields of cellular immunology and molecular biology, can solve the problem of no new coronavirus treatment drugs or vaccines being marketed, and achieve the prevention or/and treatment of new coronavirus pneumonia, high-efficiency expression, specific effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Amplification of phage display antibody library:
[0037]Inoculate 500 μL of Escherichia coli TG-1 containing Tomlinson I+J phage display antibody library (MRC HGMP Resource Center, UK) into 25 mL of 2YT medium (1.6% Tryptone, 1% Yeast Extract) containing 100 μg / mL ampicillin and 1% glucose , 0.5% NaCl), cultured at 37°C to OD 600 is 0.4, add 10 9 cfu (Colony FormationUnit) KM13 helper phage (MRC HGMP Resource Center, UK), after infection for 1 hour at 37°C, centrifuge at 3000g for 30 minutes, discard the supernatant, and use a mixture containing 100 μg / mL ampicillin, 50 μg / mL kanamycin and 0.1% Glucose 2YT medium 50mL (1.6% Tryptone, 1% Yeast Extract, 0.5% NaCl) suspend the bacteria, shake the bacteria at 30°C and 250rpm for 16 hours, centrifuge the culture solution at 5000g for 30 minutes the next day, separate and recover the supernatant 40mL , add 10mL of PEG / NaCl solution to the supernatant, mix well, place on ice for 30 minutes, centrifuge at 5000g for 30 min...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com