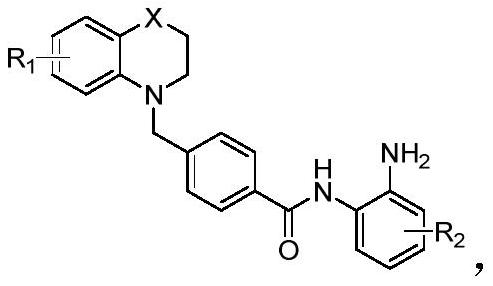
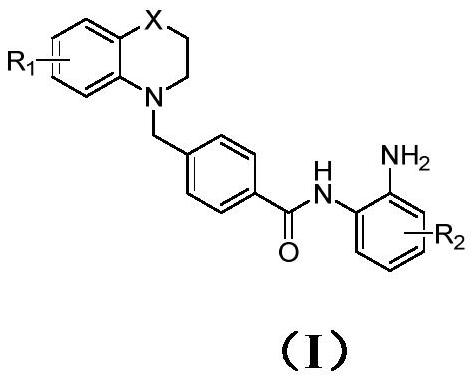
Benzamide-containing benzo six-membered heterocyclic derivative and application thereof in antitumor drugs
A benzo six-membered, benzamide technology, applied in solvates or prodrugs, anti-tumor drugs, benzo six-membered heterocyclic derivatives, can solve toxic and side effects, and lack of therapeutic targets for anti-tumor drugs etc. to achieve strong anti-tumor activity and ideal inhibition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
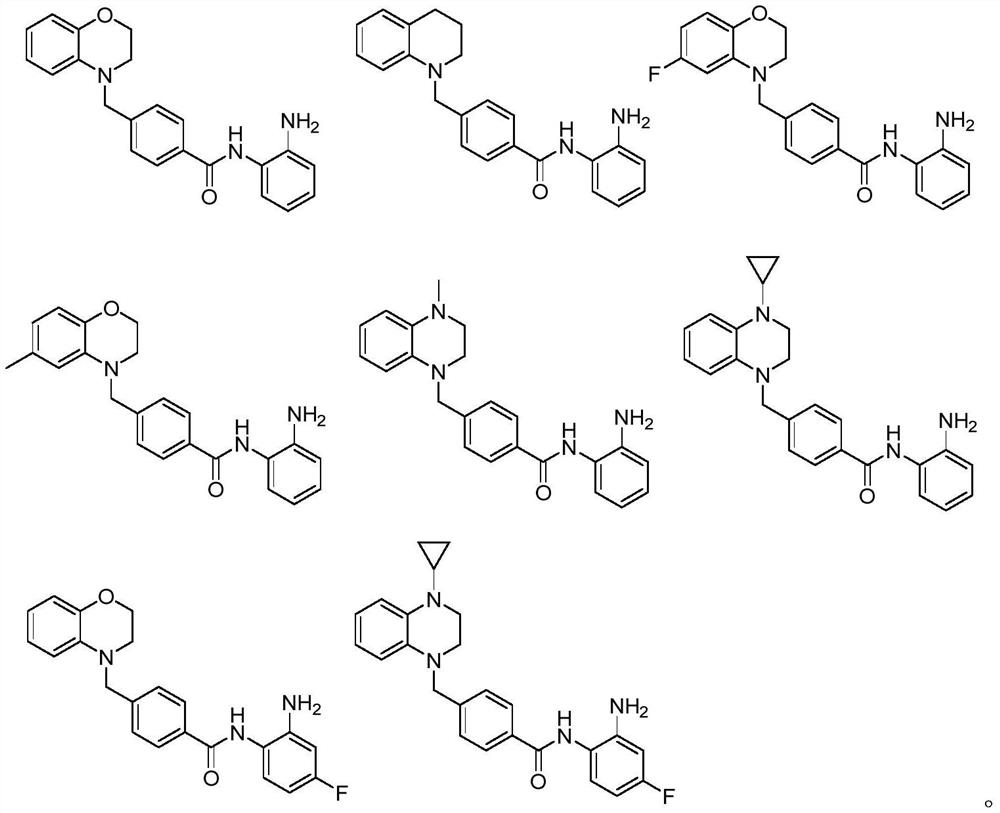
Image
Examples
Embodiment 1
[0036]
[0037] Synthesis of Step 1 Intermediate 3
[0038]Benzomorpholine (1.00 g, 7.40 mmol) and methyl 4-bromomethylbenzoate (1.69 g, 7.40 mmol) were dissolved in 30 mL of DMF, then anhydrous potassium carbonate (2.04 g, 14.8 mmol) was added, The temperature was raised to 80°C for reaction. After 6 hours of reaction, TLC detected that the reaction was complete. The reaction solution was cooled to room temperature, and the reaction solution was poured into 150 mL of water. A large amount of solid was precipitated, and 1.88 g of an off-white solid was obtained by filtration, with a yield of 89.7%.
[0039] Synthesis of Step 2 Intermediate 4
[0040] Dissolve intermediate 4 (1.88g, 6.64mmol) in 20mL of ethanol, add 10mL of 2N sodium hydroxide solution, stir at room temperature for 2h, TLC detects that the reaction is complete, distill off ethanol under reduced pressure, add water and adjust with 3N hydrochloric acid When the pH reached 4-5, a large amount of solids were pr...
Embodiment 2
[0046]
[0047] 1 H-NMR (400MHz, DMSO-d 6 )δ9.72(s,1H),7.99(d,J=7.9Hz,2H),7.33(d,J=7.8Hz,2H),7.19(d,J=7.6Hz,1H),7.12-7.10( m,1H),6.95-6.93(m,2H),6.79(d,J=8.1Hz,1H),6.65-6.61(m,3H),5.34(s,2H),4.92(s,2H),3.38 (t, J=7.2Hz, 2H), 2.78(t, J=7.1Hz, 2H), 1.96~1.91(m, 2H).
Embodiment 3
[0049]
[0050] 1 H-NMR (400MHz, DMSO-d 6 )δ9.70(s,1H),8.02(d,J=7.7Hz,2H),7.33(d,J=7.7Hz,2H),7.19(d,J=7.7Hz,1H),6.97-6.95( m,1H),6.84-6.79(m,2H),6.76(d,J=7.8Hz,1H),6.62(t,J=7.6Hz,1H),6.54(d,J=7.8Hz,1H), 5.36(s,2H),4.92(s,2H),4.15(t,J=7.2Hz,2H),3.78(t,J=7.1Hz,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com