Novel alpha O-conotoxin peptide GeXIVA new mutant as well as pharmaceutical composition and application thereof
An amino acid, cysteine technology, applied in the fields of pharmacy, biochemistry and molecular biology and neuroscience, which can solve problems such as weak blocking activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
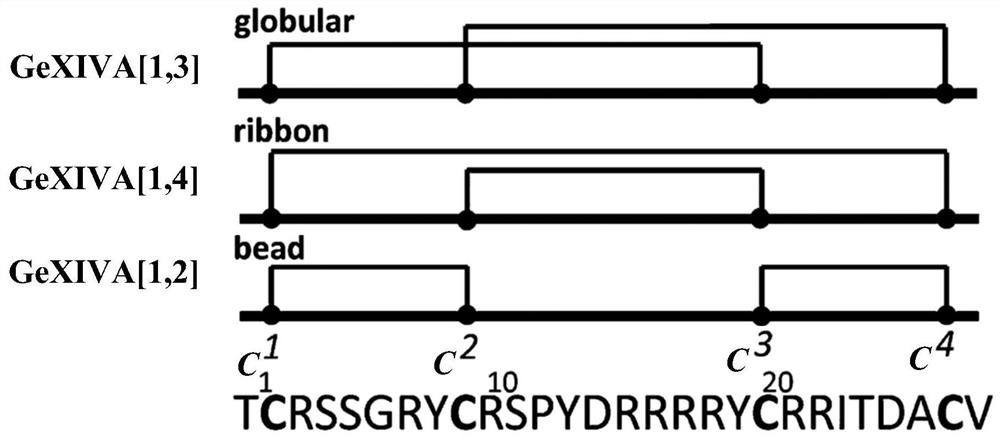
[0155] Example 1: Sequence design and artificial synthesis of a new mutant of αO-conotoxin GeXIVA
[0156] On the basis of the amino acid sequence of αO-conotoxin GeXIVA ( figure 1 , SEQ ID NOs: 1 and 25 in Table 1-2), the inventors creatively designed a series of new polypeptide mutants, the amino acid sequence of which is as SEQ ID NOs: 2-24, 25 in Table 1-7 -117 shown.
[0157] The linear peptides of the polypeptides listed in Table 1-7 were artificially synthesized by the Fmoc method. The specific method is as follows:
[0158] The resin peptide is artificially synthesized by Fmoc chemical method, and the resin peptide can be synthesized by a peptide synthesizer or manual synthesis. Except for cysteine, the remaining amino acids use standard side chain protecting groups. For a polypeptide containing 4 cysteines (Cys), if its disulfide linkage is [C1-C4, C2-C3], the -SH of the second and third cysteines (Cys) Protected with Trt (S-trityl), the -SH of the 1st and 4th ...
Embodiment 2
[0189] Example 2: A method for detecting the receptor binding activity of αO-conotoxin GeXIVA and its mutants.
[0190] References [27,37]The method in, and the instructions of the in vitro transcription kit (mMessage mMachine in vitrotranscription kit (Ambion, Austin, TX)) prepared various rat neurotype nAChRs subtypes (α3β4, α6 / α3β4, α9α10, α4β2, α4β4, α3β4, α2β2, α2β4, α7), human α9α10, and mouse muscle nAChRs (α1β1δε) cRNA, the concentration was measured by OD value under UV 260nm. Xenopus laveis oocytes (frog eggs) were dissected and collected, and cRNA was injected into the frog eggs, and the injected amount of each subunit was 5-10 ng cRNA. Frog eggs were cultured in ND-96. cRNA was injected within 1-2 days after frog egg collection, and used for voltage-clamp recordings of nAChRs within 1-4 days after injection.
[0191] One cRNA-injected frog egg was placed in a 30 μL Sylgard recording tank (diameter 4 mm × depth 2 mm), and ND96 perfusate (96.0 mM NaCl, 2.0 mM KC...
Embodiment 3
[0194] Example 3: αO-conotoxin GeXIVA[1,2] and its alanine scanning mutants are different subtypes of nAChRs blocking activity
[0195] αO-conotoxin GeXIVA[1,2] (SEQ ID NO:1, figure 1 ) and its alanine-scanning mutants (SEQ IDNOs:2-24) (Table 1) for the blocking activity of rat neurotype α9α10, α7, and mouse muscle type α1β1δεnAChRs, which are three very similar receptor subtypes , the half-blocking dose (IC 50 ) are summarized in Table 8. The ratios between the blocking activities of these mutants against the 3 subtypes are also summarized in Table 8.
[0196] Table 8: Effects of αO-conotoxin GeXIVA[1,2] (SEQ ID NO:1) and its alanine scanning mutants (SEQ IDNOs:2-24) on rat neurotype α9α10, α7, and mouse muscles Blocking activity of type α1β1δε nAChRs
[0197]
[0198] a Numbers outside brackets refer to the half-blocking dose (IC 50 ), the unit is nanomole (nM); the data in brackets is the half-blocking dose of 95% confidence interval (IC 50 ) range.
[0199] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com