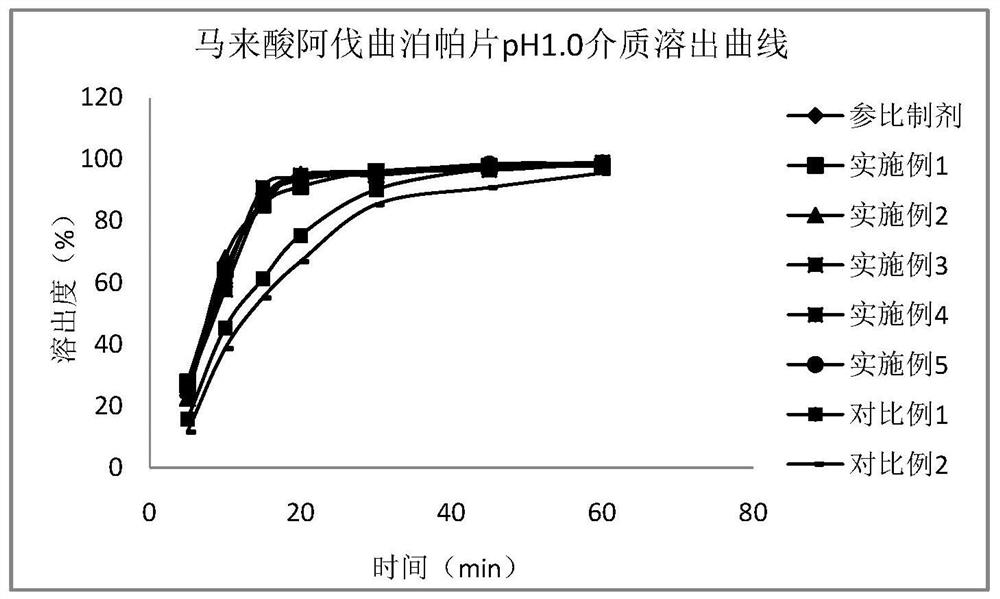
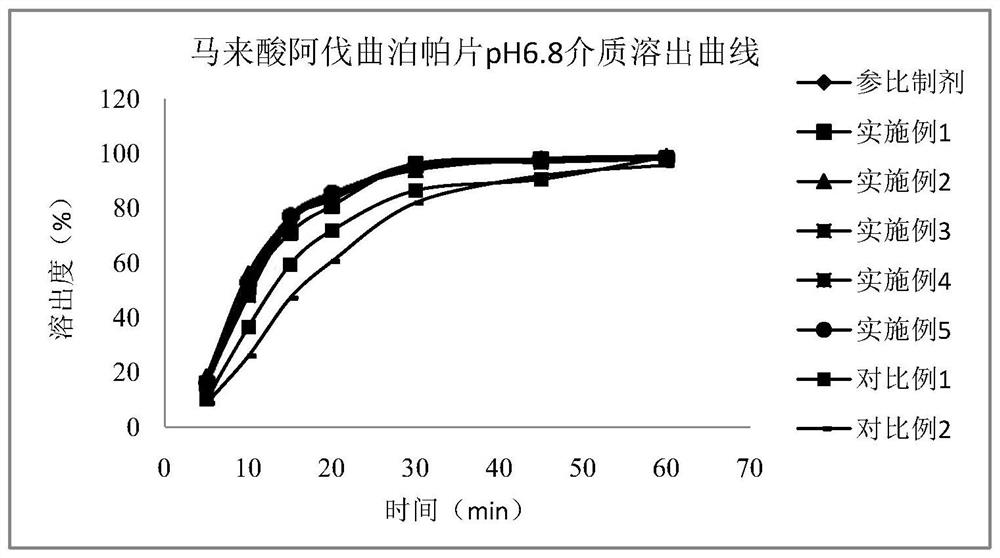
Alvatrepap maleate tablet and preparation method thereof
The invention relates to a technology for palatine tablets and maleic acid, which is applied in the field of avatrombopag maleate tablets and their preparation, which can solve problems such as poor in vitro dissolution of avatrombopag maleate and achieve process tolerance. Good, the curative effect is confirmed, and the effect of ensuring the uniformity of mixing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] A kind of alvatrombopag maleate tablet, the tablet specification is 20mg, consists of the following components:
[0039]
[0040] Appropriate amount of purified water.
[0041] Preparation:
[0042] (1) Mix 20g alvatrombopag maleate, 95g lactose monohydrate, 0.4g colloidal silicon dioxide and 3g crospovidone, and pulverize through a 16-mesh sieve;
[0043] (2) The physical mixture obtained in step (1) is mixed uniformly with 0.5g magnesium stearate, and dry granulation is carried out;
[0044] (3) Pass the granules obtained in step (2) through a 24-mesh sieve for granulation, add 5g microcrystalline cellulose, 3g crospovidone, and 0.5g magnesium stearate and mix uniformly;
[0045] (4) Add the material obtained in step (3) into a tablet press to compress the tablet, and coat it with a coating solution until the weight gain is 3%, to obtain alvatrombopag maleate tablet.
Embodiment 2
[0047] A kind of alvatrombopag maleate tablet, the tablet specification is 20mg, consists of the following components:
[0048]
[0049] Appropriate amount of purified water.
[0050] Preparation:
[0051] (1) Mix 20g avatrombopag maleate, 110g lactose monohydrate, 5g colloidal silicon dioxide and 2g croscarmellose sodium, and pulverize through a 16-mesh sieve;
[0052] (2) The physical mixture obtained in step (1) is mixed uniformly with 0.2g magnesium stearate, and dry granulation is carried out;
[0053] (3) Pass the granules obtained in step (2) through a 24-mesh sieve for granulation, add 16g of microcrystalline cellulose, 2g of croscarmellose sodium, and 0.2g of magnesium stearate and mix uniformly;
[0054] (4) Add the material obtained in step (3) into a tablet press to compress the tablet, and coat it with a coating solution until the weight gain is 5%, to obtain alvatrombopag maleate tablet.
Embodiment 3
[0056] A kind of alvatrombopag maleate tablet, the tablet specification is 20mg, consists of the following components:
[0057]
[0058] Appropriate amount of purified water.
[0059] Preparation:
[0060] (1) Mix 20g avatrombopag maleate, 110g lactose monohydrate, 1.45g silicon dioxide and 9g croscarmellose sodium, and pulverize through a 16-mesh sieve;
[0061] (2) The physical mixture obtained in step (1) is mixed uniformly with 2.5g sodium stearate fumarate, and dry granulated;
[0062] (3) Pass the granules obtained in step (2) through a 24-mesh sieve for granulation, add 16g of microcrystalline cellulose, 3g of croscarmellose sodium, and 2.5g of sodium fumarate stearate and mix uniformly;
[0063] (4) Add the material obtained in step (3) into a tablet press to compress the tablet, and coat it with a coating solution until the weight gain is 5%, to obtain alvatrombopag maleate tablet.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com