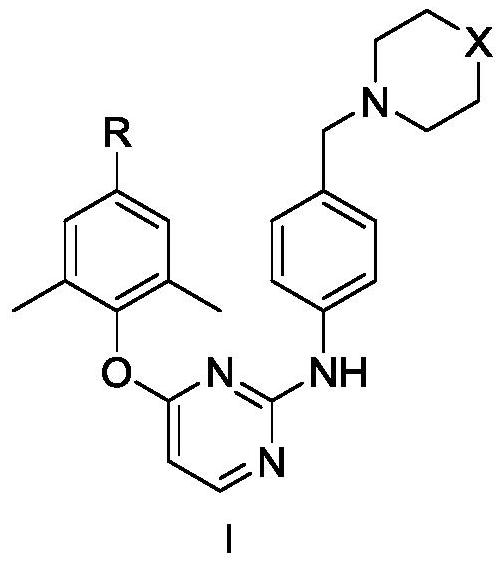
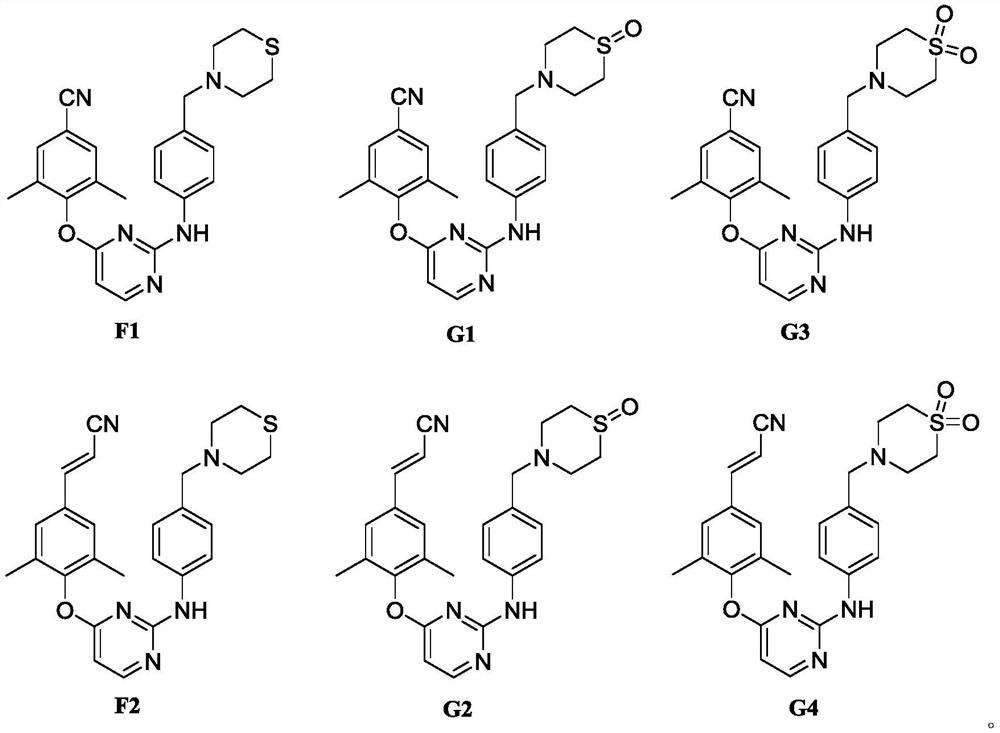
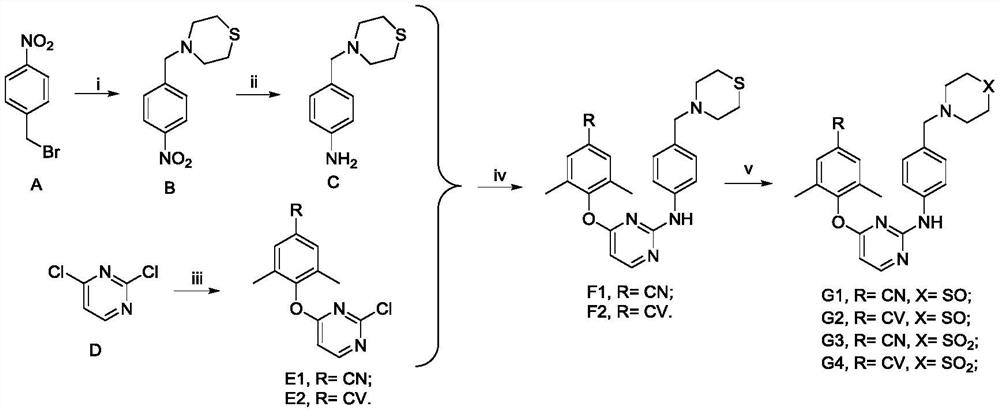
Diarylpyrimidine derivative containing six-membered nitrogen heterocyclic ring as well as preparation method and application of diarylpyrimidine derivative
A technology of diarylpyrimidine and nitrogen heterocycle, which is applied in the directions of medical preparations containing active ingredients, organic chemistry, pharmaceutical formulations, etc., can solve the problems of large oral dose, low oral bioavailability, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: Preparation of 4-(4-nitrobenzyl)thiomorpholine (B)
[0030]
[0031] 4-Nitrobenzyl bromide A (2.16g, 0.01mol) and thiomorpholine (1.03g, 0.01mol) were dissolved in dichloromethane (20mL), and triethylamine (1.21g, 0.012mol). The resulting mixture was stirred at room temperature until completion as monitored by TLC. Quench the reaction with 30 mL of water, then wash with saturated brine, separate the organic layer, and use anhydrous Na 2 SO 4 Dry on celite, filter, and concentrate under reduced pressure. The residue was recrystallized from ethyl acetate / petroleum ether to obtain intermediate B. Pale yellow solid, yield: 92.1%. 1 H NMR (400MHz, DMSO-d 6 )δ8.19(d, J=8.6Hz, 2H, Ph-H), 7.59(d, J=8.6Hz, 2H, Ph-H), 3.64(s, 2H, CH 2 ), 2.67–2.60 (m, 8H, thiomorpholine-H). ESI-MS:m / z 239.08(M+H) + ,C 11 h 14 N 2 o 2 S(238.08).
Embodiment 2
[0032] Embodiment 2: the preparation of 4-(thiomorpholine methyl) aniline (C)
[0033]
[0034] The intermediate 4-(4-nitrobenzyl)thiomorpholine B (1.0 g) was dissolved in 20 ml of absolute ethanol, then stannous chloride dihydrate (5.0 g) was added. The reaction mixture was stirred at room temperature under nitrogen until complete as monitored by TLC. 2 mol / L NaOH was added to the mixture to adjust the pH to 7. The resulting white solid was filtered and washed with ethyl acetate. The filtrate was added with saturated sodium chloride solution and ethyl acetate. Use anhydrous Na 2 SO 4The organic layer was dried, filtered, concentrated under reduced pressure, and dried in vacuo to finally obtain the intermediate 4-(thiomorpholinemethyl)aniline C. Used directly in the next step without further purification. Yellow solid, yield: 52.2%. ESI-MS:m / z 208.95(M+H) + ,241.03(M+H) + , C 11 h 16 N 2 S(208.10).
Embodiment 3
[0035] Example 3: Preparation of 4-((2-chloropyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile (E1)
[0036]
[0037] 2,4-Dichloropyrimidine D (1.49 g, 0.01 mol) and potassium carbonate (1.66 g, 0.012 mol) were dissolved in dimethylformamide (20 mL), and 4-hydroxy-3,5 - Dimethylbenzonitrile (1.47 g, 0.01 mol) and stirred at 50 °C for 4 h until the reaction was complete. Ice water (200 mL) was added, and the mixture was extracted with ethyl acetate (3 x 50 mL). The combined organic layers were washed with saturated brine, using anhydrous Na 2 SO 4 Drying, filtration and concentration under reduced pressure and finally recrystallization using ethyl acetate and petroleum ether afforded the pure intermediate 4-((2-chloropyrimidin-4-yl)oxy)-3,5-dimethylbenzonitrile E1. White solid, yield: 88.4%. 1 H NMR (400MHz, DMSO-d 6 )δ8.70(d, J=5.7Hz, 1H, pyrimidine-H), 7.75(s, 2H, Ph-H), 7.32(d, J=5.7Hz, 1H, pyrimidine-H), 2.10(s, 6H, Ph-CH 3 ×2). ESI-MS:m / z 259.97(M+H) + ,C 13 h 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com