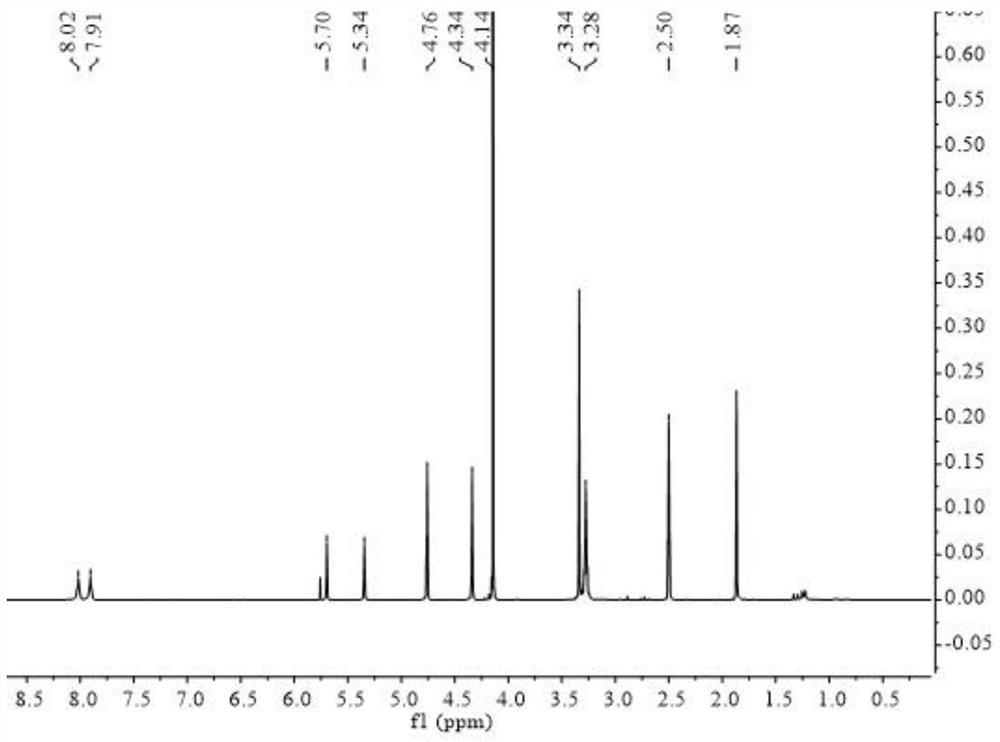
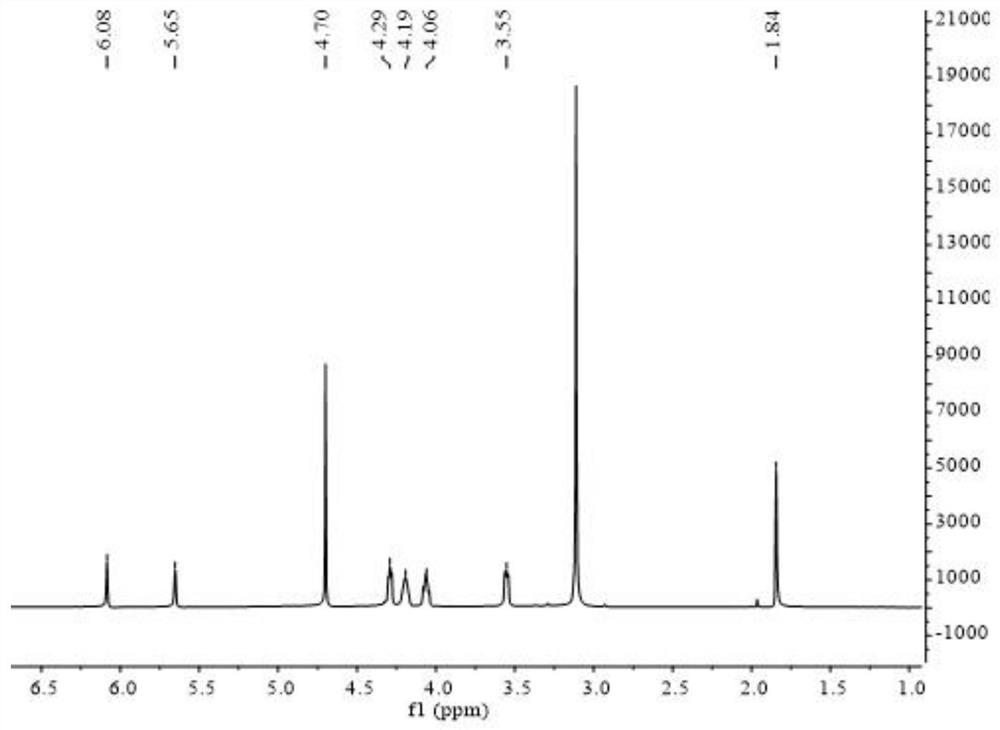
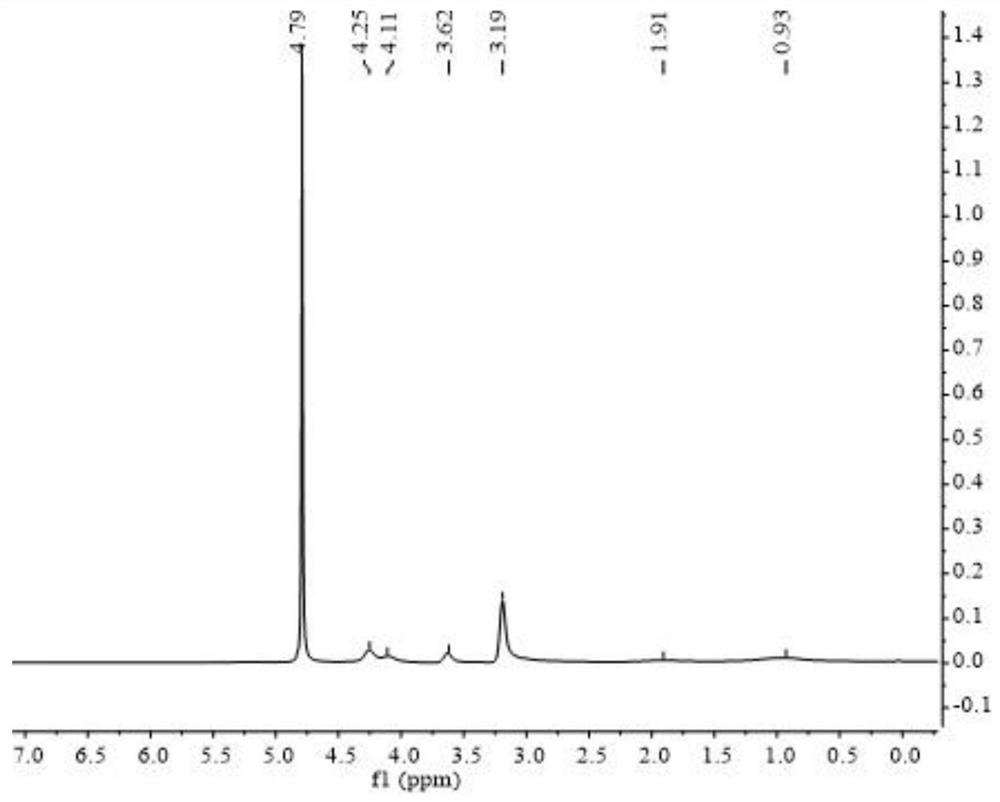
Polymer electrolyte containing zwitter-ionic groups and redox groups and preparation method of polymer electrolyte
A zwitterion and polymer technology, applied in the field of electrochemistry, can solve the problems of inability to meet the use of solid-state supercapacitors, and achieve the effects of excellent electrochemical performance and simple preparation method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A method for preparing a polymer electrolyte, comprising the steps of:
[0042] S1, prepare dimethyl sulfoxide (DMSO) / absolute ethanol=9 / 1 solution (S), pass nitrogen gas to remove oxygen for 2 hours in advance, and set aside;
[0043] S2, weigh 68.41mg of monomer II-1 and 295.27mg of monomer III-1 in reaction test tubes, deoxygenate them with double row tubes, add 1.5mL of S, and dissolve them into solution M;
[0044] S3, weigh 1.97mg of BPO in the reaction test tube, use a double-row tube to deoxygenate it, add 0.5ml of S, and dissolve it into a solution R;
[0045] S4, adding the solution R into the solution M, heating to 70°C, reacting for 8 hours, filtering, dialysis of the filtrate for 48 hours, and freeze-drying for 48 hours to obtain the polymer electrolyte I-1.
Embodiment 2
[0047] A method for preparing a polymer electrolyte, comprising the steps of:
[0048] S1, prepare dimethyl sulfoxide (DMSO) / absolute ethanol=8 / 1 solution (S), pass nitrogen gas to remove oxygen for 2 hours in advance, and set aside;
[0049] S2, weigh 73.91mg of monomer II-2 and 319.01mg of monomer III-2 in reaction test tubes, deoxygenate them with double row tubes, add 1.5mL of S, and dissolve them into solution M;
[0050] S3, weigh 2.13mg of BPO in the reaction test tube, use a double-row tube to deoxygenate it, add 0.5ml of S, and dissolve it into a solution R;
[0051] S4, add the solution R into the solution M, heat to 70° C., react for 12 hours, filter, dialyze the filtrate for 48 hours, and freeze-dry for 72 hours to obtain the polymer electrolyte I-2.
Embodiment 3
[0053] A method for preparing a polymer electrolyte, comprising the steps of:
[0054] S1, prepare dimethyl sulfoxide (DMSO) / absolute ethanol = 19 / 1 solution (S), pass nitrogen gas to remove oxygen for 2 hours in advance, and set aside;
[0055] S2, weigh 68.41mg of monomer II-3 and 590.54mg of monomer III-3 in reaction test tubes, deoxygenate them with double row tubes, add 1.5mL of S, and dissolve them into solution M;
[0056] S3, weigh 3.61mg of BPO in the reaction test tube, use a double-row tube to deoxygenate it, add 0.5ml of S, and dissolve it into a solution R;
[0057] S4, add the solution R into the solution M, heat to 60° C., react for 36 hours, filter, dialyze the filtrate for 36 hours, and freeze-dry for 24 hours to obtain the polymer electrolyte I-3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com