Engineering streptomycete for producing daunorubicin and construction method of engineering streptomycete
A technology of engineering streptomyces and daunorubicin, applied in the biological field, can solve problems such as difficult industrial output levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Example 1: In Vitro Fishing of Daunorubicin Synthetic Gene Cluster
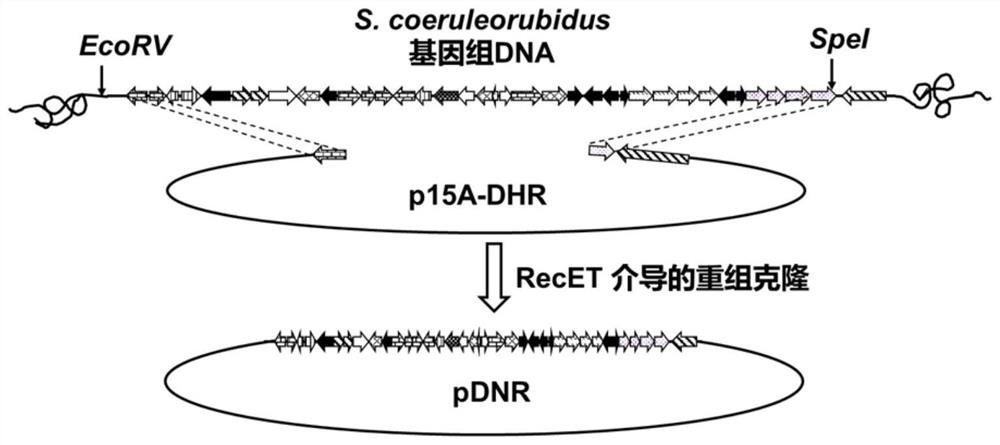
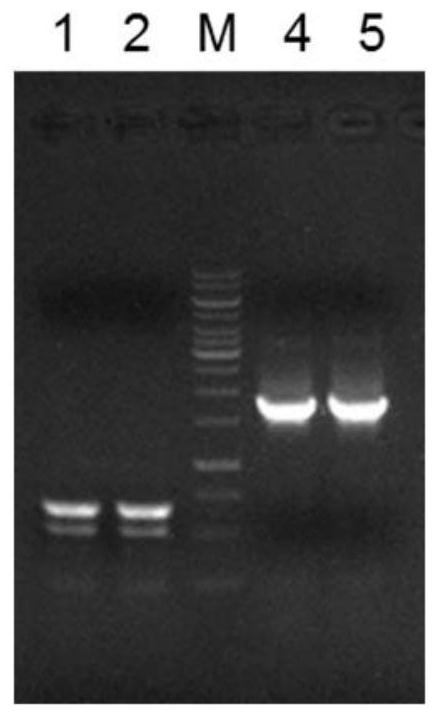
[0057] The invention utilizes the Red / ET DNA recombination technology to catch the daunorubicin synthetic gene cluster in vitro from the genomic DNA of Streptomyces coelicolor (CICC11043) capable of synthesizing daunorubicin, and insert it into the cluster suitable for the integration of Streptomyces. The type vector p15A-DHR was used to obtain the recombinant vector pDNR containing daunorubicin. The present invention is based on the Red / ETDNA recombination system (Wang H, Li Z, Jia R, et al. RecET direct cloning and Redαβ recombineering of biosyntheticgene clusters, large operons or single genes for heterologous expression[J].nature protocols, 2016, 11(7 ): 1175.) Design scheme, construct the schematic diagram as figure 1 shown.
[0058] The specific construction process is:
[0059] (1.1) Using the Streptomyces coelicolor genomic DNA as a template, use the primer pair DNR01-F / R to amplify the homo...
Embodiment 2
[0074] Example 2: Transformation of the promoter of the pathway-specific activator gene
[0075] The present invention uses the high-efficiency constitutive promoter kasOp* suitable for Streptomyces to replace the promoter of the daunorubicin synthesis pathway-specific activator gene dnrI on the pDNR carrier, so that the activator can be efficiently expressed in Streptomyces, Thereby activating the biosynthetic pathway of daunorubicin.
[0076] The specific modification steps are as follows:
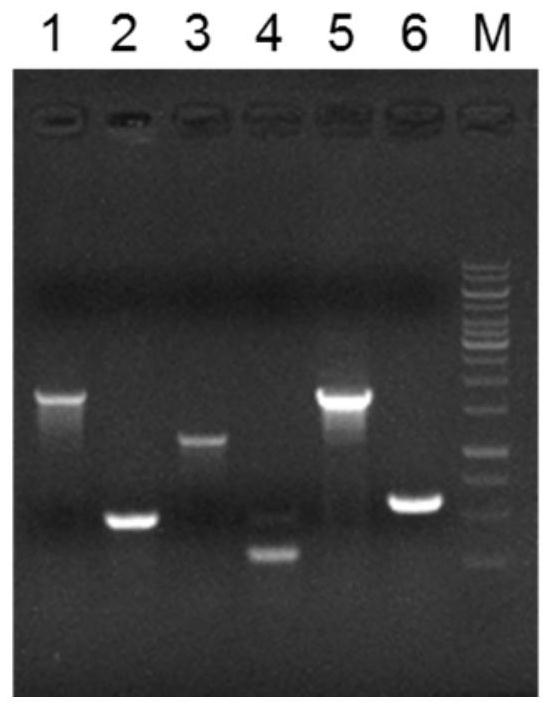
[0077] (2.1) Using the recombinant vector pDNR as a template, use the primer pair DNR04-F / R and DNR05-F / R to amplify the upstream 195bp region and the downstream 196bp length of the dnrI promoter region (-1 to -174 bases) respectively The region of is used as the homology arm;
[0078] The sequences of the primers DNR04-F, DNR04-R, DNR05-F, and DNR05-R are shown in SEQ ID NO.14, SEQ ID NO.15, SEQ ID NO.16, and SEQ ID NO.17. :
[0079] (2.2) Use primers DNR04-F and DNR05-R to fuse the...
Embodiment 3
[0083] Example 3: Targeted knockout of branch-related genes in the synthesis of daunorubicin
[0084] The present invention uses the Red / ET DNA recombination technology to synthesize the by-product paromomycin-related methyltransferase genes dnrH and dnrX in the daunorubicin synthesis gene cluster, and to catalyze daunorubicin to reversely generate 13-dihydro The reductase gene dnrU of daunorubicin is targeted to be knocked out to improve the synthesis efficiency of daunorubicin and eliminate the interference of impurities in the subsequent preparation process.
[0085] (3.1) Use the primer pair DNR06-F / R and DNR07-F / R to amplify the region of the upstream 183bp length of the dnrH open reading frame and the downstream region of the 173bp length as homology arms;
[0086] The sequences of the primers DNR06-F, DNR06-R, DNR07-F, and DNR07-R are shown in SEQ ID NO.18, SEQ ID NO.19, SEQ ID NO.20, and SEQ ID NO.21.
[0087] (3.2) using primers DNR06-F and DNR07-R to obtain the DNA ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com