Coronavirus detection product and application thereof
A coronavirus and product technology, applied in the field of molecular biology, can solve the problems of increasing the time for patients to seek medical treatment, high cost of patient testing, missed detection of coronavirus, etc., and achieve the effect of reducing medical treatment time, easy operation and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0121] Embodiment 2 The present invention compares with other commercialization extraction kits extraction effect
[0122] 1. Experimental method
[0123] 1.1 Detection object
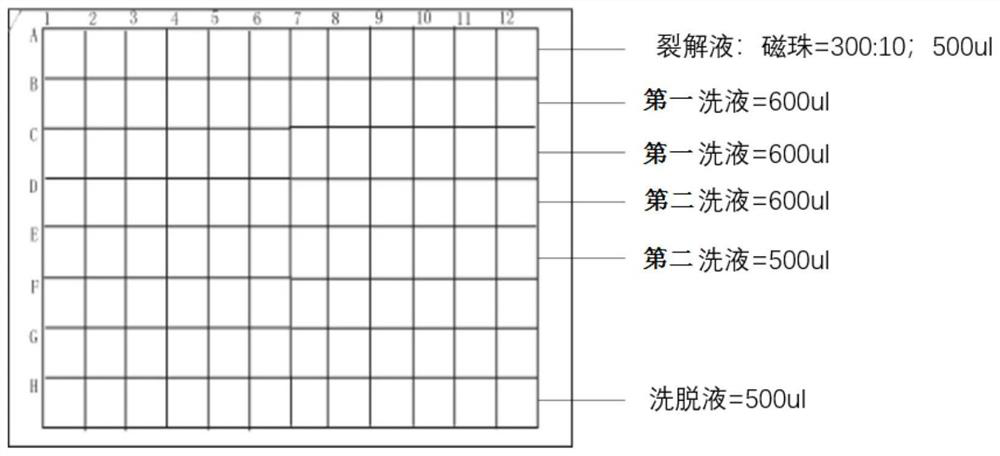
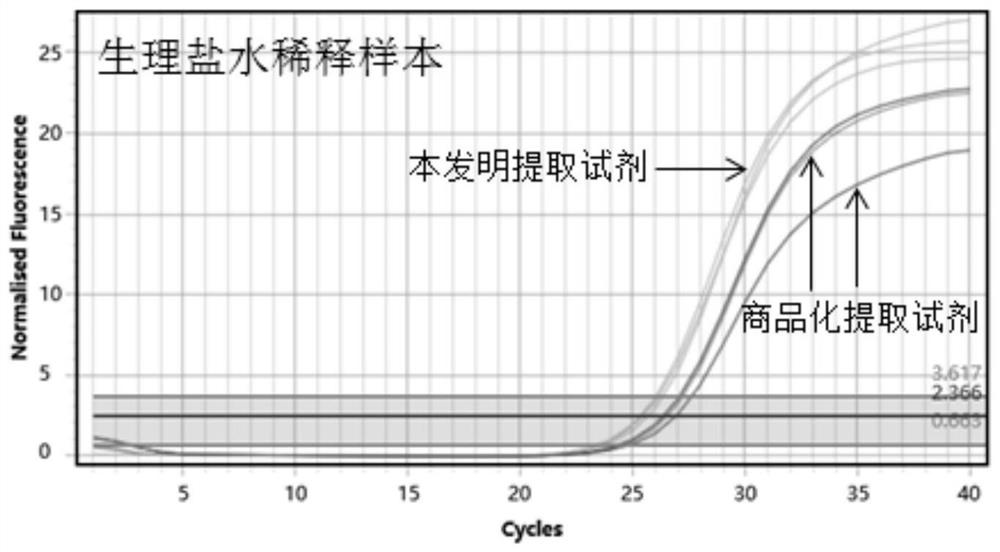
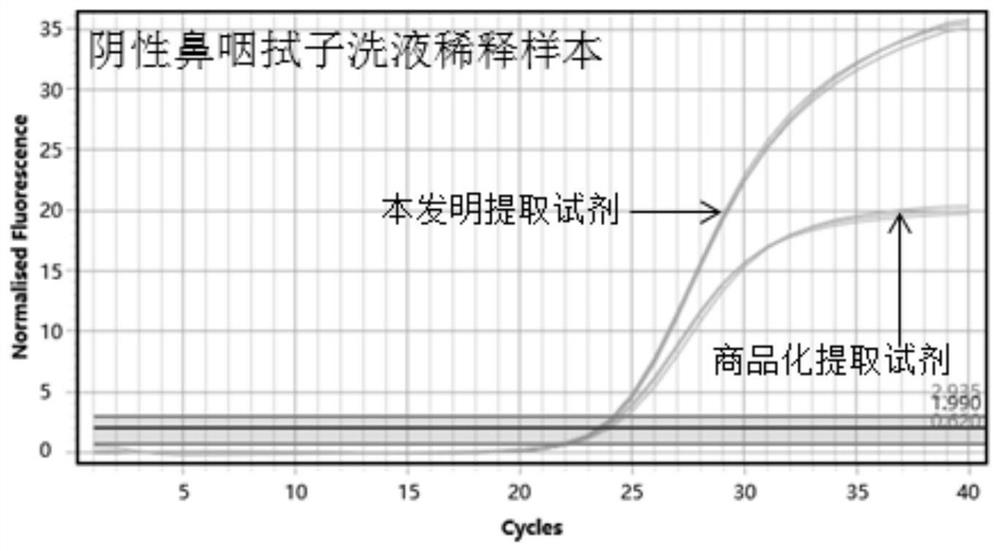
[0124] Construct a high-concentration Coronavirus simulated positive sample. The simulated positive sample is composed of the synthetic product of the new coronavirus genome sequence (as shown in SEQ ID NO.10 and SEQ ID NO.11) and the background matrix of normal human exfoliated cells. Take a high-concentration Coronavirus simulated sample. For positive samples, three gradients were diluted with Coronavirus-negative nasopharyngeal swab wash samples and normal saline, respectively. The three concentration samples diluted in the coronavirus negative nasopharyngeal swab wash sample are respectively defined as XL-1, XL-2, and XL-3, and the three concentration samples diluted in normal saline are respectively defined as XN1, XN2, and XN3.
[0125] 1.2 Inactivation of samples
[0126] Take the above sampl...
Embodiment 3
[0150] Application of the Coronavirus joint detection kit in embodiment 3 saliva and sputum
[0151] 1. Experimental method
[0152]1.1 Detection object
[0153] The saliva and sputum obtained from 4 clinical cases were tested for Coronavirus nucleic acid. These 4 samples have been identified as 2 Coronavirus positive samples and 2 Coronavirus negative samples by culture method in the hospital, which are numbered S1, S2, S3 and S4 in sequence.
[0154] 1.2 Sample inactivation and nucleic acid extraction
[0155] Take the above samples and add 1.5mL virus inactivator respectively, shake and mix well, and let stand at room temperature for 30min.
[0156] Use the self-contained extraction reagent of the present invention to carry out the extraction of 4 examples of saliva and sputum samples (same as 1.3 in Example 2). Add 1 μL of internal standard to row A of the preloaded plate, and then add S1, S2, S3, S4, negative quality control or positive quality control to each well in ...
Embodiment 4
[0165] Coronavirus detection kit performance evaluation in embodiment 4 sputum
[0166] 1. Experimental method
[0167] 1.1 Detection object
[0168] (1) Sensitivity analysis
[0169] Take 1 copy of Coronavirus simulated positive sample, dilute it 10 times with sample diluent to form 5 gradients, numbered S1, S2, S3, S4 and S5 in sequence, add negative quality control and positive quality control, nucleic acid extraction and detection .
[0170] The detection method is as follows: Take 30 μL of the PCR detection mixture, mix it with 0.5 μL of PCR enzyme, shake and mix for a few seconds, centrifuge at 3000 rpm for a few seconds, take 30 μL of the above-mentioned mixed liquid and put it in a PCR tube, and then add 20 μL of the sample, negative quality control or Add the nucleic acid extraction product of the positive quality control product into the PCR reaction tube, cover the PCR reaction tube cap, and proceed to the PCR amplification reaction immediately.
[0171] The cyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com