Freeze-dried formulation for gas-filled microvesicles
A freeze-dried, microbubble technology that is used in preparations for in vivo testing, echo/ultrasonic imaging agents, pharmaceutical formulations, etc., and can solve problems such as low percentages and vials failing to pass acceptability tests.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Determination of the percentage of folded chains by MDSC
[0056] a. Calibration of equipment and systems
[0057] All MDSC experiments were performed on a Tzero TM technology (which allows direct measurement of heat capacity) and Option (which allows the superimposition of sinusoidal temperature fluctuations on conventional linear temperature ramps) was implemented on a DSC-Q2000 system (TA Instruments, New Castle, DE USA).
[0058] Conveniently operate from -90°C to 550°C using the Refrigerated Cooling Attachment (RCS90) with a two-stage refrigeration system.
[0059] Data acquisition and processing were performed with the help of the Universal Analysis Software package.
[0060] A Tzero aluminum crucible (ref 901683.901) and a Tzero aluminum lid (ref 901671.901) (both obtained from TAinstruments) were used to contain the sample to be examined and the crucible was sealed by a Tzero press (ref 901600.901).
Embodiment 2
[0086] Preparation of freeze-dried cake
[0087]The procedure for preparing the lyophilized cake essentially followed that described in the examples of WO 94 / 09829. Briefly, first dissolve DSPC, DPPG-Na and PA in a weight ratio of 4.75 / 4.75 / 1 in hexane / ethanol (8 / 2, v / v) at a concentration of about 5 g / L, and The solvent was evaporated. The residue was mixed with PEG4000 in a weight ratio of about 0.017:1, the mixture was dissolved in tert-butanol at about 60 °C, and the clear solution was filled into individual DIN8R vials (corresponding volume containing about 25 mg of the mixture). The vials were then rapidly cooled at -45°C before a final lyophilization step. At the end of lyophilization, use SF under normal pressure 6 Ambient conditions of saturated lyophilizate, and seal described vial (comprising with SF 6 solid lyophilized cake in contact).
Embodiment 3
[0090] Vial / Dry Cracker Inspection
[0091] Each batch obtained by the preparation method described in Example 2 was inspected as follows to check whether there was any substandard freeze-dried cake.
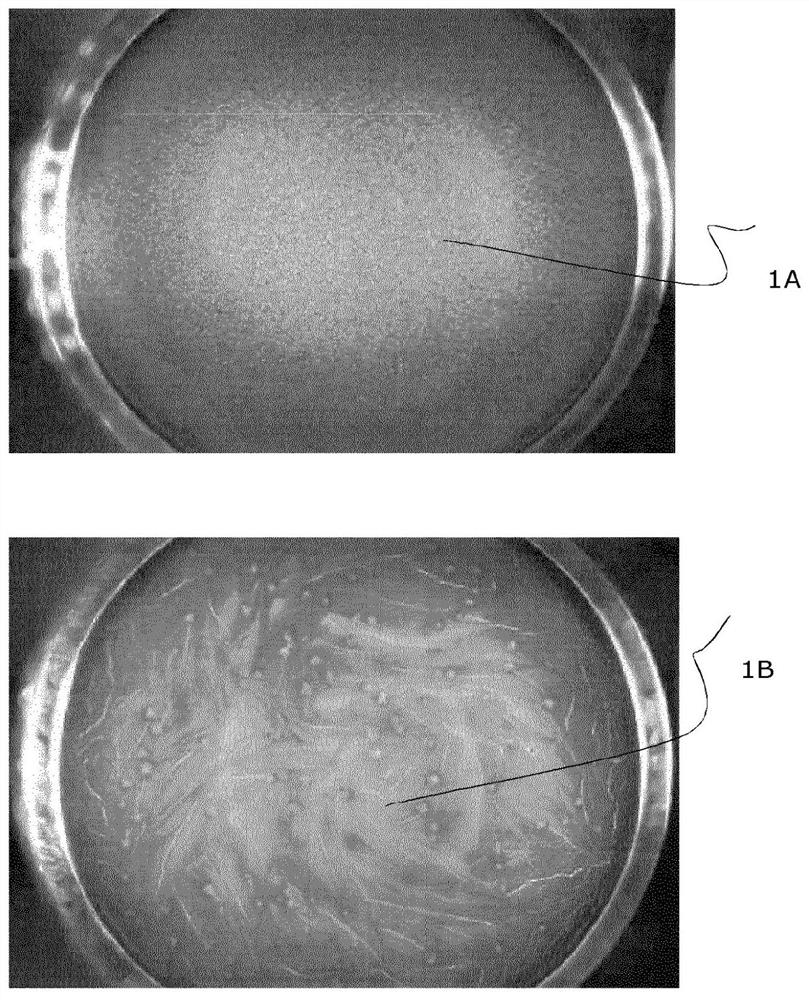
[0092] Each batch of vials was inspected using a Seidenader M10063 semi-automatic machine according to the process described above and classified as "acceptable vials" ie containing smooth dry cake (SCV, e.g. figure 1 a) or "rejected vials" that contain rough dry cake (RCV, such as figure 1 shown in b).
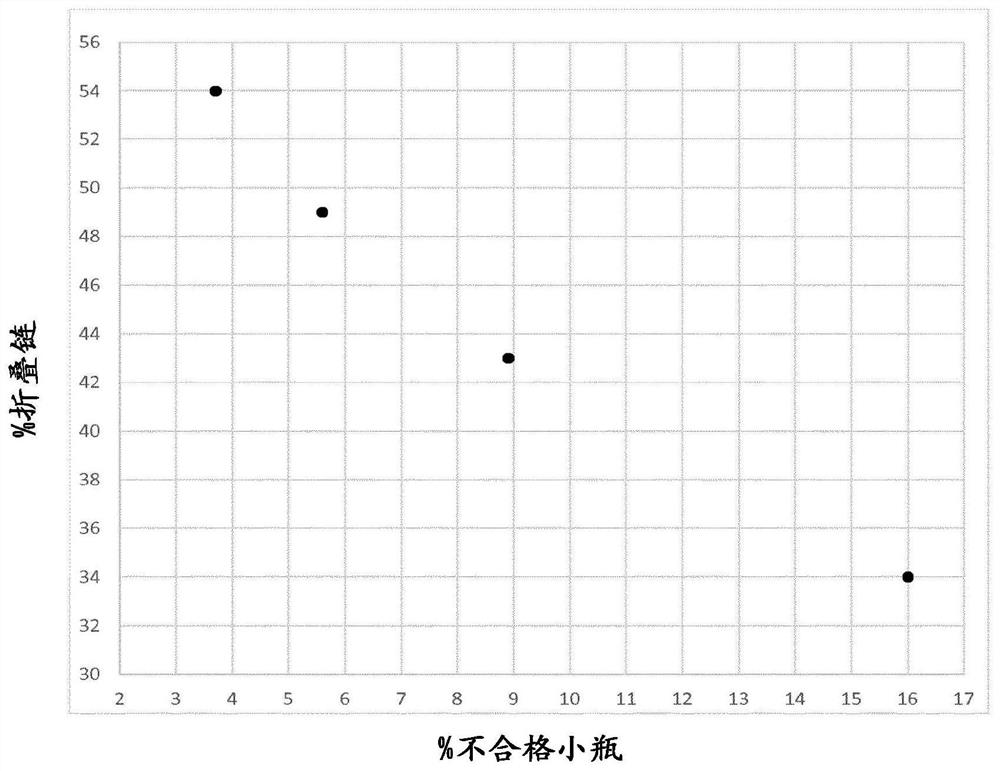
[0093] The inspection results implemented according to the above process are shown in Table 7 below and recorded in figure 2 middle.
[0094] Table 7: Relationship between volume of rejected vials and % folded chains in PEG4000
[0095] production batch % of defective vials PEG4000 folded chain % Batch 1 (comparative example) 16 34 batch 2 8.9 43 batch 3 5.6 49 batch 4 3.7 54
[0096] From the results shown in Table 7, it can ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com