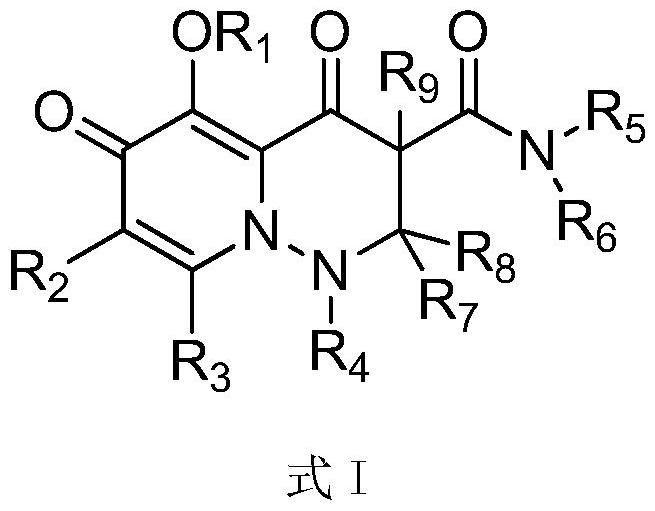
2-substituted-5-hexahydropyridazinone-4-carboxylic acid amine derivative and application thereof
A compound and hydrate technology, applied in the field of 2-substituted-5-hexahydropyridazinone-4-carboxylic acid amine derivatives and anti-influenza drugs, can solve the problems of low immunity and low efficacy in children
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] Example 1: N-ethyl-1-(7,8-difluoro-6,11-dihydrodibenzo[b,e]thiazepine-11-yl)-5-hydroxyl-4,6- Dioxo-2,3,4,6-tetrahydro-1H-pyrido[1,2-b]pyridazine-3-carboxamide
[0164] Obtained by the following reaction formula:
[0165] Step 1) Synthesis of compound 1-2
[0166]
[0167] Add isobutyl chloroformate ( 92.9mg, 680.0umol, 89.3uL, 1.10eq). The mixture was stirred at 0-5°C for 30 mins. Add ethylamine (27.9mg, 618.2umol, 40.5uL, 1.00eq) to the mixture at 0~5°C. The mixture was stirred at 20-30°C for 60 mins. TLC (EtOAc:MeOH=10:1, R f (R1)=0.50) showed complete consumption of Compound 1 and formation of multiple spots. The reaction solution was concentrated to dryness, the residue was washed successively with 2-MeTHF (1 mL) and saturated brine, and the organic layer was concentrated to dryness. The crude product was purified by prep-TLC (EtOAc:MeOH=10:1), and LCMS showed the expected m / z to obtain compound 1-2 (0.100g, 231.8umol, 37.5%yield) as a yellow oil, which ...
Embodiment 2
[0174] Example 2: 1-(7,8-difluoro-6,11-dihydrodibenzo[b,e]thiazin-11-yl)-5-hydroxy-4,6-dioxo-2 ,3,4,6-tetrahydro-1H-pyrido[1,2-b]pyridazine-3-carboxamide
[0175] Obtained by the following reaction formula:
[0176] Step 1) synthesis of compound 2-2
[0177]
[0178] Compound 2-1 (200.0g, 812.3mmol, 1.00eq), EtI (228.0g, 1.46mol, 116.9mL, 1.80eq), DBU (185.5 g, 1.22mol, 183.7mL, 1.50eq) in DMF (1000mL) Stir at 20~30℃ for 1hrs.TLC (PE:EA=1:1,R f = 0.60) showed that the reaction was complete. The mixture was introduced into 4L of water and extracted with EA (1000mL*3). The organic layer was washed with saturated brine (1000 mL*2) and concentrated to dryness to obtain compound 2-2 (190.0 g, 692.8 mmol, yield 85.3%) as a yellow oil. HNMR conforms to the expected structure. 1 H NMR: (400MHz, CHLOROFORM-d) δ7.74(d, J=5.6Hz, 1H), 7.51-7.44(m, 2H), 7.39-7.29(m, 3H), 6.46(d, J=5.6Hz ,1H),5.36-5.23(m,2H),4.33(q,J=7.2Hz,2H),1.31(t,J=7.1Hz,3H)
[0179] Step 2) Synthesis of ...
Embodiment 3
[0206] Example 3: N-isopropyl-1-(7,8-difluoro-6,11-dihydrodibenzo[b,e]thiepine-11-yl)-5-hydroxy-4,6 -Dioxo-2,3,4,6-tetrahydro-1H-pyrido[1,2-b]pyridazine-3-carboxamide
[0207] Obtained by the following reaction formula:
[0208] Step 1) Synthesis of compound 3-1
[0209]
[0210] Compound 1-1 (0.300g, 741.81umol, 1.00eq), DMF (3mL), i-PrNH 2 (219.2mg, 3.71mmol, 318.7uL, 5.00eq), HATU (338.5mg, 890.2umol, 1.20eq) were stirred at 20~30°C for 1hr. LCMS showed the expected m / z. The mixture was diluted with 2-MeTHF (3mL) , saturated saline nd washed with sat.aq.NaCl (2mL*3). The organic layer was concentrated to dryness to obtain compound 3-1 (0.300 g, 673.4 umol, 90.8% yield) as a yellow oil, which was used for the next reaction. MS(ESI, m / z): 446.2[(M+H) + ].
[0211] Step 2) Synthesis of Compound 3-2
[0212]
[0213] Compound 3-1 (300mg, 673.34umol, 1.00eq), A (178.0mg, 673.4umol, 1.00eq), T 3 A solution of P (3.21 g, 5.04 mmol, 3.00 mL, purity.0% purity, 7.49...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com