A kind of ultra-low molecular weight heparin sodium and preparation method thereof
A technology of heparin sodium and molecular weight, applied in the field of biomedicine, can solve the problem of high cost, and achieve the effect of convenient operation, strong practicability and simple method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
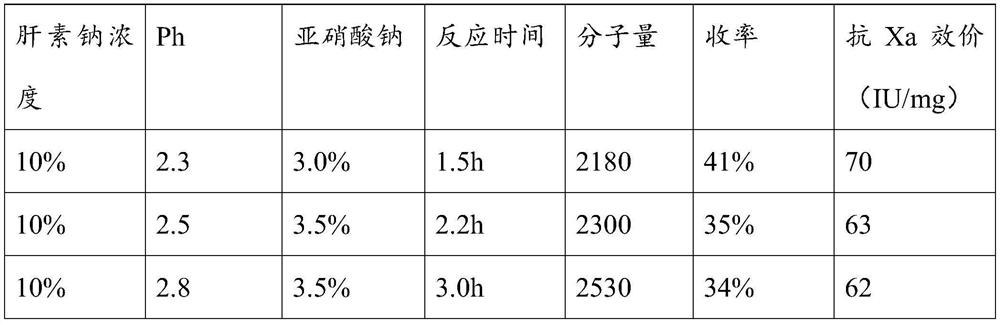
[0026] A preparation method of ultra-low molecular weight heparin sodium, comprising the following steps:
[0027] (1) Preparation of solution A: 3-6% sodium chloride (W / V) contains 2-3% sodium nitrite (calculated according to the weight of heparin sodium) aqueous solution (adjust the pH of the solution to 2-3 )
[0028] (2) Take the fine heparin sodium, make it into a 5-15% heparin sodium aqueous solution, add the A solution while stirring at room temperature, and constantly monitor and adjust the pH of the solution to stabilize it at PH to 2.0 to 3.0. After confirming the reaction (after generating a large number of bubbles), use a 3000 Dalton membrane for ultrafiltration, add 4% sodium chloride solution containing 2-3% sodium nitrite aqueous solution while filtering, and keep the volume of the reaction solution in the retention zone unchanged. , maintain the PH of the liquid in the interception zone at 2.0-3.0, and stop the ultrafiltration after no bubbles are produced in ...
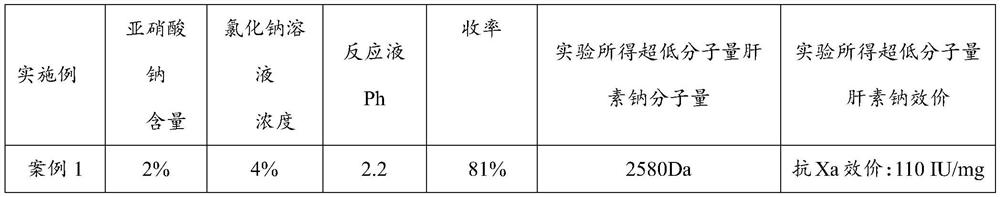
Embodiment 1
[0037] (1) get 100g of fine heparin sodium, be made into 10% heparin sodium aqueous solution 1000ml, add 4% sodium chloride containing 2% sodium nitrite (sodium nitrite is calculated according to the weight of the heparin sodium) aqueous solution (the solution of PH is adjusted to 2.2) at room temperature, add 6mol / L hydrochloric acid while stirring to adjust the pH to 2.2, after the reaction produces bubbles, carry out ultrafiltration with a 3000-dalton membrane, add 4% of sodium chloride while filtration Sodium nitrate (sodium nitrite is calculated according to the weight of heparin sodium) aqueous solution, keep the volume of the reaction solution in the interception zone unchanged, and maintain pH 2.2 throughout the process, which should be adjusted at any time. The ultrafiltration was stopped after no bubbles were generated in the reaction solution and no heparin sodium was detected in the solution.
[0038] (2) collect the filtrate obtained in step (1), and adjust the pH...
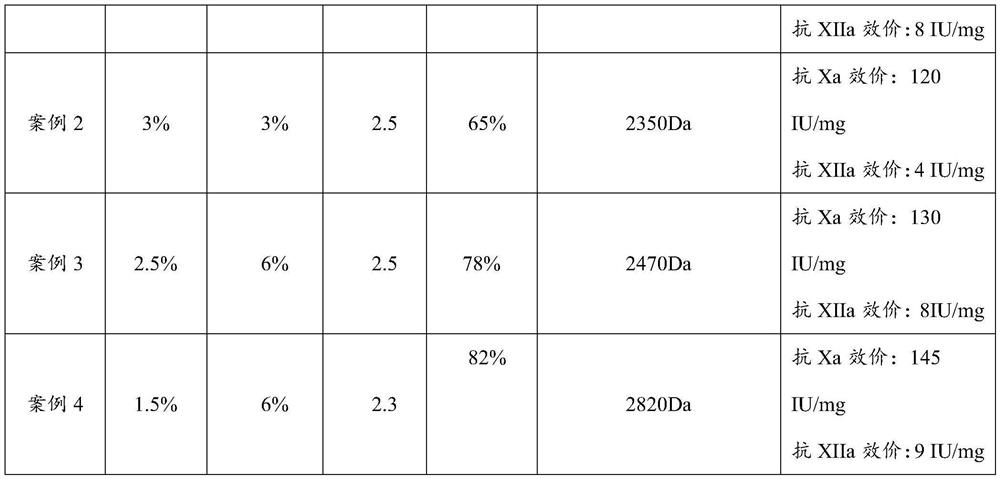
Embodiment 2
[0059] (1) take 100g of fine heparin sodium and dissolve it into 1000ml of 15% aqueous solution, add 3% sodium chloride containing 3% sodium nitrite aqueous solution (the pH of the solution is adjusted to 2.5), add 6mol / L hydrochloric acid while stirring at room temperature Adjust the pH to 2.5, use a 3000 Dalton membrane for ultrafiltration after the reaction bubbles are generated, add 3% sodium chloride solution containing 3% sodium nitrite aqueous solution while filtering, and keep the pH to 2.5 to keep the reaction in the trapping zone The volume of the solution remained unchanged, and the ultrafiltration was stopped after no bubbles were generated in the reaction solution and no heparin sodium was detected in the solution.
[0060] (2) Collect the filtrate in (1), and adjust the pH to 7.0, then add 1 g of solid sodium borohydride (the amount added is 1% by weight of heparin sodium) to the filtrate after reduction reaction for 12 hours, use 1000 Dalton The membrane was sub...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com