Methods of treating heavy menstrual bleeding
A severe, endometrial technology applied in the field of GnRH receptor antagonists, which can solve the problems of administration inconvenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0246] Example 1: Summary of Efficacy and Safety Findings from a Phase 2 Study Completed with Endometriosis Subjects
[0247] effect
[0248] Six phase 2 randomized, double-blind, placebo- and / or active-controlled, parallel-group, multiple-dose studies evaluating elagolix as management of endometriosis-associated pain were completed. Dysmenorrhea (DYS), nonmenstrual pelvic pain (NMPP), dyspareunia, and general pelvic pain efficacy were assessed using a battery of instruments.
[0249] Other efficacy measures included quality of life and use of analgesics to manage endometriosis pain.
[0250] In these phase 2 studies, inclusion criteria were similar and aimed to select premenopausal women aged 18 to 49 years by visual inspection (laparoscopic or laparotomy during the 5 to 8 year screening process) Confirmed endometriosis, experiencing moderate to severe endometriosis-related pain. Women with normal menstrual cycles and no overt fibroids or pelvic lesions were also included. ...
example 2
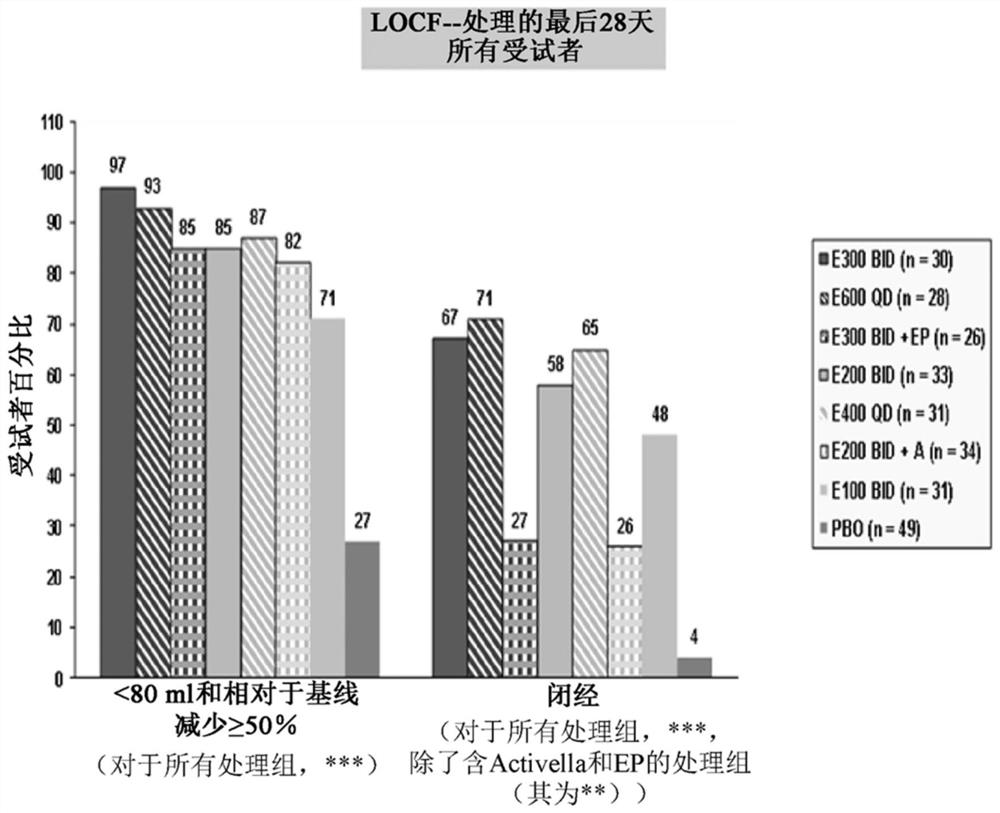
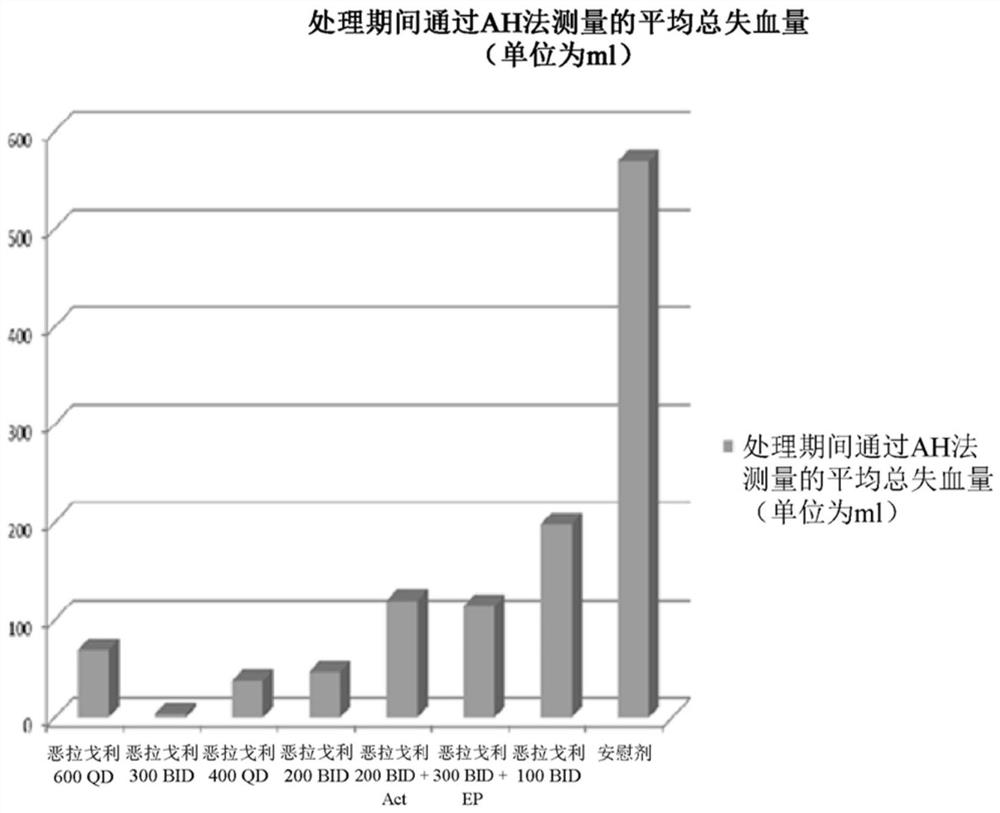
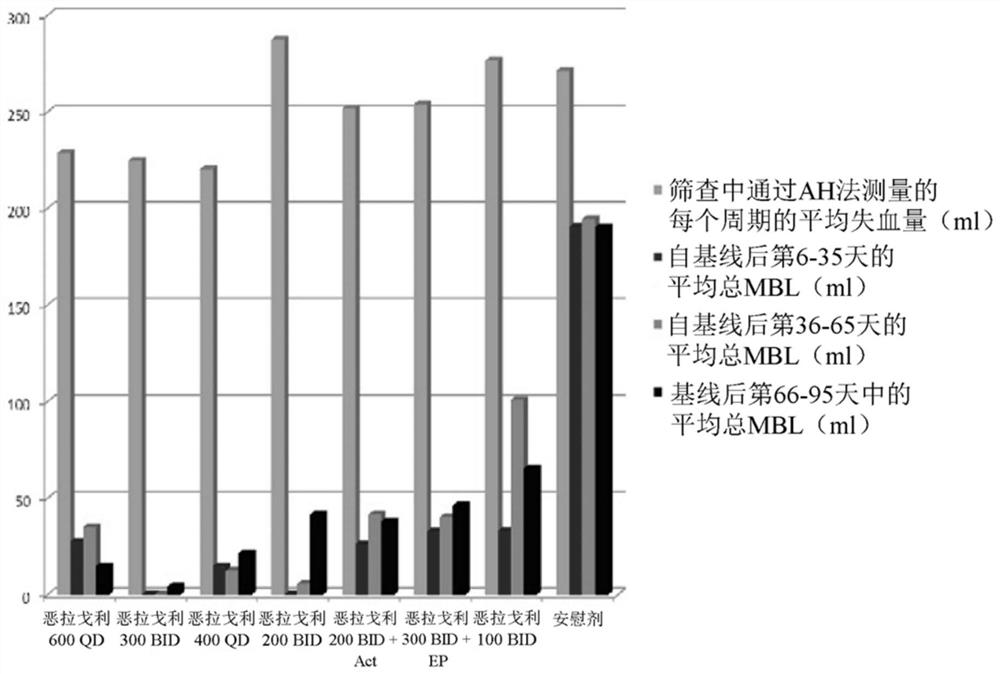
[0261] This example is a phase 2a, multicentre, double-blind, placebo-controlled, randomized group trial (N=280) in premenopausal women with uterine fibroids, with a treatment duration of 3 months, evaluating combination versus no joint Safety and efficacy of administered elagolix.
[0262] It evaluated 6 doses of elagolix (100mg BID, 200mg BID, 200mg BID plus low dose (combination of 0.5mg estradiol and 0.1mg norethindrone acetate), 300mg BID, 300mg BID plus 1.0mgEstrace and 200mg periodic Prometrium (collectively referred to as "EP"), 400mg QD and 600QD) compared to placebo (PBO) in patients Safety and efficacy of reducing fibroid-related uterine bleeding and reducing fibroid volume and uterine volume in premenopausal women aged 20 to 49 years with heavy menstrual bleeding (HMB; blood loss >80 mL per menstrual cycle). The study involved the following six (6) groups:
[0263] Group 1: elagolix 200 mg BID or placebo (PBO).
[0264] Group 2: elagolix 300mg BID or placebo....
example A-1
[0288] Example A-1: Efficacy and safety of elagolix in a subgroup of women with uterine fibroids and non-overt adenomyosis
[0289] Adenomyosis is an estrogen-dependent disorder in which benign endometrial tissue grows within the muscular tissue of the uterus and is associated with heavy menstrual bleeding (HMB) and dysmenorrhea. Adenomyosis occurs when endometrial tissue, usually the lining of the uterus, exists within and grows into the muscular wall of the uterus. With each menstrual cycle, the displaced endometrial tissue continues to act as it always did, i.e. thicken, rupture and bleed. This can lead to an enlarged and painful uterus and increased menstrual flow. Symptoms most often begin in the late reproductive years after childbirth. The cause of adenomyosis is unknown, but the disease usually disappears after menopause. For women who experience severe discomfort from adenomyosis, certain treatments may help, but hysterectomy is the only cure. Sometimes adenomyo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com