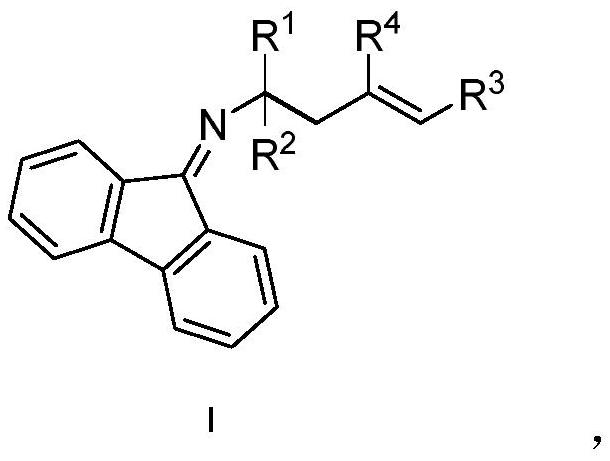
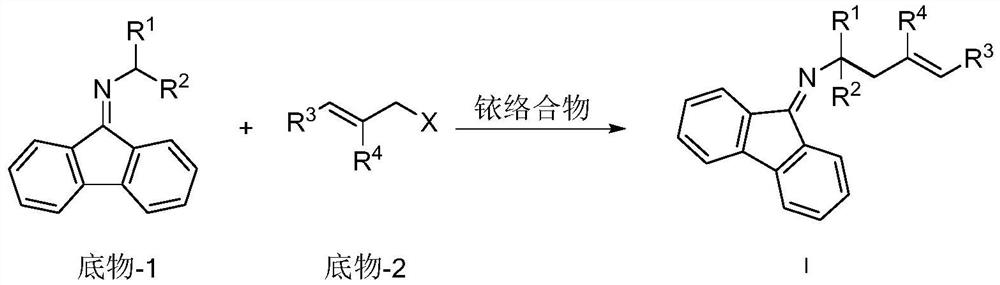
Chiral alpha-polysubstituted-alpha-fluorine-containing homoallylamine compound, preparation method and application thereof
A high-allylamine, multi-substitution technology, applied in the field of chemical medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] 1) preparation of
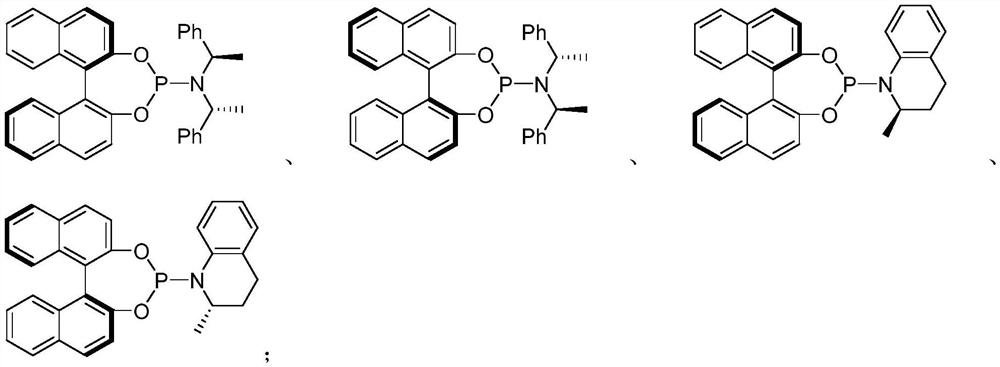
[0067] Add 0.005mmol[Ir(COD)Cl] to 25mL reaction tube 2 , 0.010mmol (S, S, S)-L1, 0.5mL deoxygenated THF and 0.5mL deoxygenated n-propylamine, reacted at 50°C for 30 minutes, and evaporated the solvent under reduced pressure to obtain an iridium catalyst. Under the protection of nitrogen at 25°C, add 2mL of toluene, and then add 0.20mmol N-2,2,2-trifluoroethylfluorenone imine, 0.22mmol cinnamyl methyl carbonate and 0.20mmol 1,8-diazabis Cycloundec-7-ene, react at 25°C. The reaction was monitored by thin-layer chromatography. After the reaction was completed, the solvent was removed under reduced pressure and purified by silica gel (treated with triethylamine) column chromatography to obtain the product.
[0068] 2) preparation of
[0069] Dissolve the product obtained in step 1) in 1 mL of dichloroethane, add a methanol solution of hydroxylamine acetate (0.5M, 1 mL, prepared in methanol from hydroxylamine hydrochloride, sodium hydroxide and ac...
Embodiment 2
[0071] 1) preparation of
[0072] Add 0.005mmol[Ir(COD)Cl] to 25mL reaction tube 2 , 0.010mmol (S, S, S)-L1, 0.5mL deoxygenated THF and 0.5mL deoxygenated n-propylamine, reacted at 50°C for 30 minutes, and evaporated the solvent under reduced pressure to obtain an iridium catalyst. Under nitrogen protection at 25°C, 2 mL of toluene was added, followed by 0.20 mmol of N-2,2,2-trifluoroethylfluorenoneimine, 0.22 mmol of p-methylphenylallyl methyl carbonate and 0.20 mmol of 1,8- Diazabicycloundec-7-ene, reacted at 25°C. The reaction was monitored by thin-layer chromatography. After the reaction was completed, the solvent was removed under reduced pressure and purified by silica gel (treated with triethylamine) column chromatography to obtain the product.
[0073] 2) preparation of
[0074] Dissolve the product obtained in step 1) in 1 mL of dichloroethane, add a methanol solution of hydroxylamine acetate (0.5M, 1 mL, prepared in methanol from hydroxylamine hydrochloride, s...
Embodiment 3
[0076] 1) preparation of
[0077] Add 0.005mmol[Ir(COD)Cl] to 25mL reaction tube 2 , 0.010mmol (S, S, S)-L1, 0.5mL deoxygenated THF and 0.5mL deoxygenated n-propylamine, reacted at 50°C for 30 minutes, and evaporated the solvent under reduced pressure to obtain an iridium catalyst. Under nitrogen protection at 25°C, 2 mL of toluene was added, followed by 0.20 mmol of N-2,2,2-trifluoroethylfluorenoneimine, 0.22 mmol of m-methylphenylallyl methyl carbonate and 0.20 mmol of 1,8- Diazabicycloundec-7-ene, reacted at 25°C. The reaction was monitored by thin-layer chromatography. After the reaction was completed, the solvent was removed under reduced pressure and purified by silica gel (treated with triethylamine) column chromatography to obtain the product.
[0078] 2) preparation of
[0079] Dissolve the product obtained in step 1) in 1 mL of dichloroethane, add a methanol solution of hydroxylamine acetate (0.5M, 2 mL, prepared in methanol from hydroxylamine hydrochloride, s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com