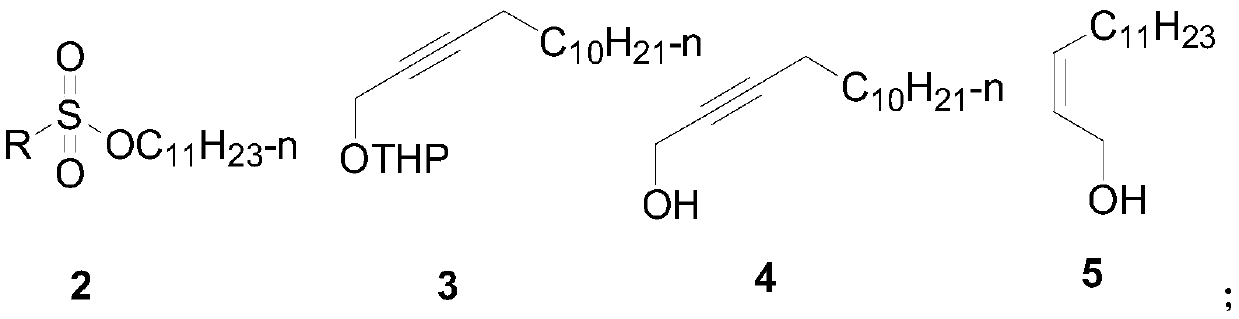
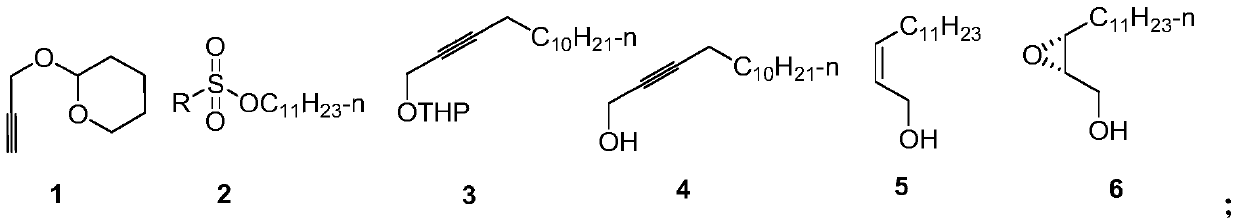
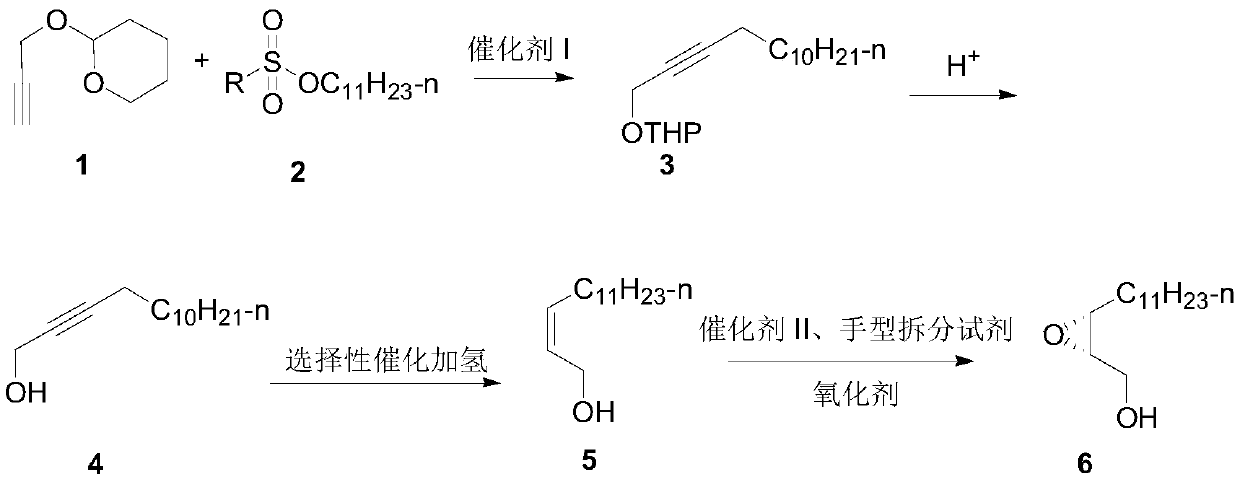
Synthesis method of hyphantria cunea sex pheromone intermediate
A technology of American white moth and synthetic method, applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of high economic cost, difficult to guarantee safety, difficult to operate, etc., and achieve the effect of good corresponding selectivity and short route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: the synthesis of intermediate 3
[0041]
[0042] In a 250mL three-necked flask, add tetrahydropyran propargyl alcohol (that is, 2-(2-propargyloxy) tetrahydropyran, 1.4g, 10mmol), 40mLTHF, N 2 Protected, put in -30~-20 ℃ low-temperature tank and drop the temperature to -30 ℃, stir and cool, add n-BuLi (4.4mL 2.5M, 11mmol), after one hour of reaction, add p-toluenesulfonate undecane ( 2.6 g, 11 mmol), naturally raised to room temperature for 12 h, added 2 mL of water to quench the reaction, and then used.
Embodiment 2
[0043] Embodiment 2: the synthesis of intermediate 4
[0044]
[0045] Add p-toluenesulfonic acid (5mmol) or concentrated hydrochloric acid 0.5mL to the crude product solution of intermediate 3 in the above-mentioned example 1, heat and reflux for 5 hours, add water to terminate the reaction, remove THF by rotary evaporation, extract with ethyl acetate, dry and concentrate, and column The product was obtained by chromatography (eluent: V (ethyl acetate) / V (petroleum ether) = 1 / 10), 1.87 g, yield 89%, purity greater than 98%.
[0046] 1 H NMR (400MHz, CDCl 3 )δ 4.25 (s, 2H), 2.21 (ddd, J = 7.1, 5.0, 2.1 Hz, 2H), 1.54-1.21 (m, 19H), 0.88 (t, J = 6.8 Hz, 3H).
Embodiment 3
[0047] Embodiment 3: the synthesis of intermediate 5
[0048]
[0049] After Lindlar catalyst (2g) was suspended in triethylamine (20ml) for 1h, 2-tetradecyn-1-alcohol (21g, 100mmol) dissolved in triethylamine (80ml) was added to the above-mentioned catalytic system, Hydrogenation at room temperature under normal pressure, after the hydrogen absorption is completed, filter, and the filtrate is dried and concentrated to obtain a colorless liquid, which can be directly used for the next step reaction, 19g, yield 90%. The Z / E value is greater than 99:1.
[0050] 1 H NMR (600MHz, CDCl 3 )δ5.67-5.50 (m, 2H), 4.22 (d, J = 6.4Hz, 2H), 2.09 (dd, J = 14.6, 7.4Hz, 2H), 1.63 (d, J = 41.7Hz, 1H), 1.43-1.21 (m, 18H), 0.90 (t, J=7.0Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com