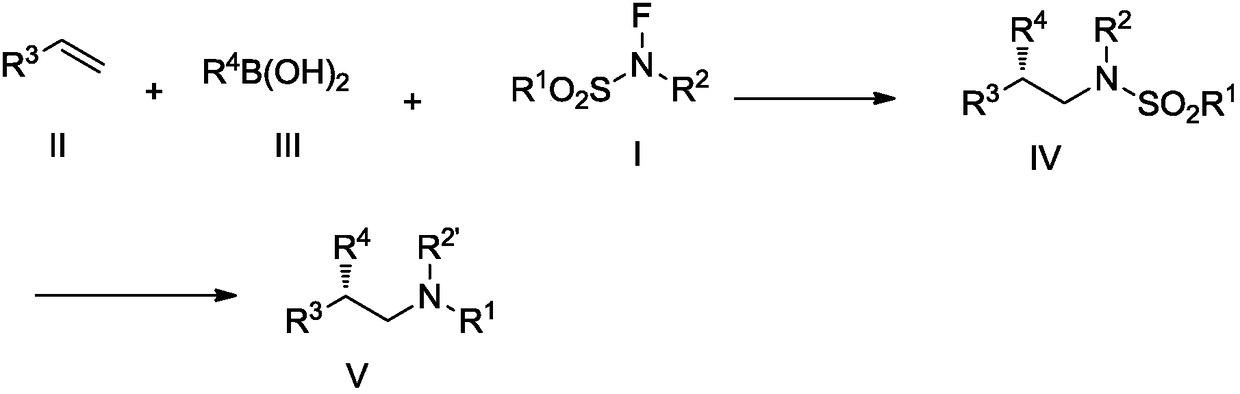
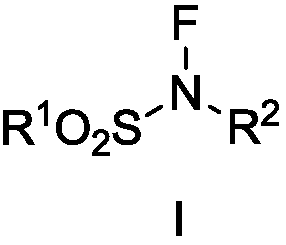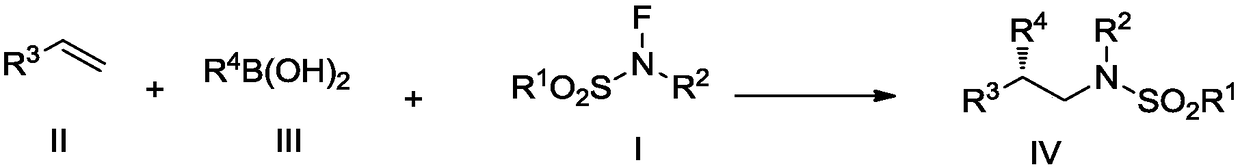A kind of fluorine nitrogen type amination reagent, its preparation method and application
A fluorine-nitrogen, amination technology, used in the preparation of sulfonic acid amides, chemical instruments and methods, preparation of organic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076]
[0077] In a 500mL three-necked flask, N-methylbenzenesulfonamide (13.5g, 78.9mmol) was dissolved in FCCl 3 / HCl 3 (200mL, v / v 1:1) mixed solvent, after cooling to -78°C, F 2 / N 2 (2:8) The gas is slowly passed into the reaction solution (1-2 bubbles / minute). After 3 hours of reaction, TLC showed that most of the starting material had been consumed. Access to N 2 Half an hour later, after the reaction returned to room temperature, the reaction solution was passed through a short column of silica gel, CH 2 Cl 2 Rinse, concentrate the filtrate, and separate by flash column chromatography (petroleum ether / ethyl acetate=20:1) to obtain 6.7 g (45% yield) of a colorless liquid. R f =0.5 (petroleum ether / ethyl acetate=10:1). 1 H NMR (400MHz, CDCl 3 )δ7.97(d, J=8.0Hz, 2H), 7.77(t, J=8.0Hz, 1H), 7.64(t, J=8.0Hz, 2H), 3.17(d, J=31.6Hz, 3H) . 13 C NMR (100MHz, CDCl 3 )δ135.0, 131.0, 130.1, 129.3, 40.8 (d, J=12.2Hz). 19 F NMR (376MHz, CDCl 3 )δ-37.46(q,J=31.6Hz).H...
Embodiment 2
[0079]
[0080]Weigh NaH (3.6g, 60mmol, 60wt% in mineral oil) into a 500mL round bottom bottle, add anhydrous CH 2 Cl 2 (250mL), stirred into a paste. Under the protection of Ar gas, a dichloromethane solution (50 mL) of N-alkylbenzenesulfonamide (30 mmol) was added dropwise, and a large amount of gas was generated during the dropwise addition. After the dropwise addition was completed, stirring was continued at room temperature for 30 minutes. N-fluorobisbenzenesulfonimide (NFSI, 56 g, 180 mmol) was added into the reaction system, and stirred at room temperature for 6 hours. The reaction solution was slowly poured into ice water to quench unreacted NaH. Separate the organic phase with a separatory funnel and the aqueous phase with CH 2 Cl 2 (200mL×3) extraction, anhydrous MgSO 4 The organic phase is dried and filtered. After the filtrate was concentrated, it was separated by flash column chromatography (PE / EA=20:1) to obtain the target product.
[0081]
[0082]...
Embodiment 3
[0095]
[0096] Step 1: In a 100mL sealed tube, ligand L* (82.0mg, 0.20mmol) and Cu(CH 3 EN) 4 PF 6 (37.0mg, 0.10mmol) was dissolved in DMA / CH under the protection of argon 2 Cl 2 (1:4, 20 mL), after stirring for 0.5 h, the resulting colorless solution was ready for use.
[0097]Step 2: In a sealed 10 mL tube, add the compound represented by formula III (0.40 mmol, 2.0 equiv) and anhydrous LiOtBu (8.0 mg, 0.10 mmol, 0.5 equiv). Under the protection of argon, in the reaction tube cooled to -20°C, add the above copper catalyst solution (2.0mL), the compound (0.20mmol, 1.0equiv) shown in formula II, and the fluorine nitrogen shown in formula I Type amination reagent (0.4mmol, 2.0equiv), the reaction solution turned blue. The reaction was stirred at -10°C. After the specified time, the reaction solution was diluted with 20 mL of ethyl acetate and washed with water (10 mL×3). The organic phase was washed with anhydrous MgSO 4 Drying, filtration, after the filtrate is conc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com