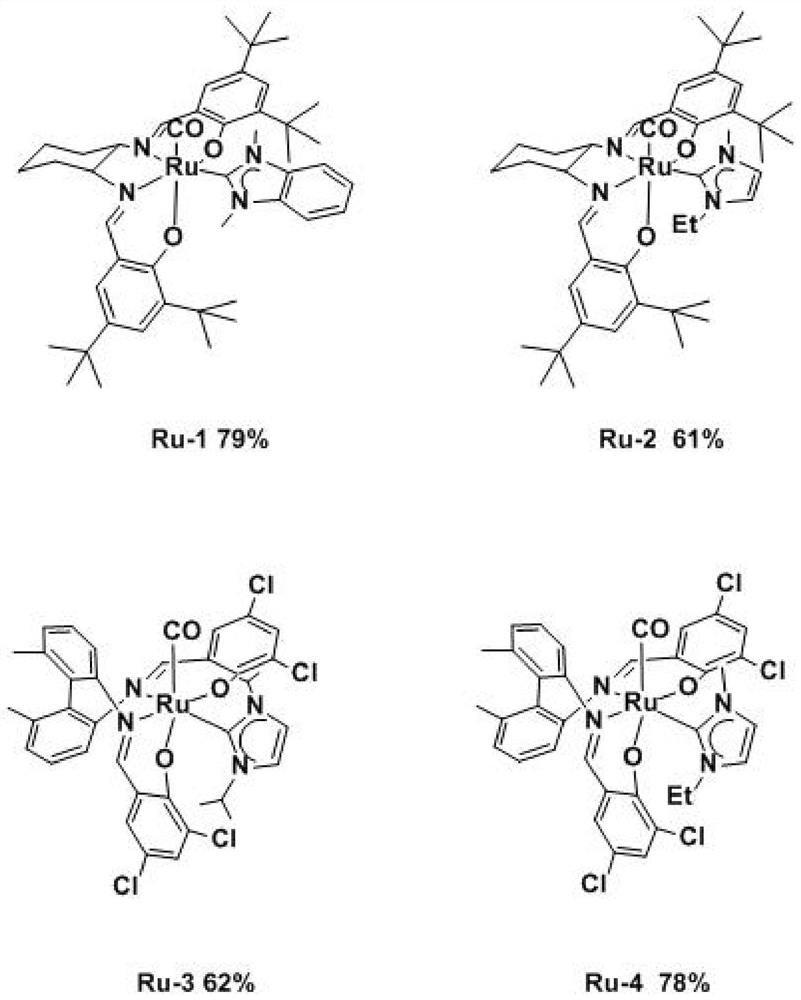
A kind of preparation method of cis-ruthenium-salen nitrogen heterocyclic carbene
A technology of nitrogen heterocyclic carbene and nitrogen protection, which is applied in chemical instruments and methods, ruthenium organic compounds, compounds containing elements of group 8/9/10/18 of the periodic table, etc., can solve the problem of low yield and synthetic method Complicated problems, to achieve the effect of increasing productivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of preparation method of Cis-ruthenium-salen nitrogen heterocyclic carbene, the synthetic steps are as follows:
[0037] (1) Synthesis of Precursor A
[0038] Precursor A was synthesized according to the method disclosed in J.Am.Chem.Soc.2009, 131, 4405-4417, and the yield was 35%.
[0039]
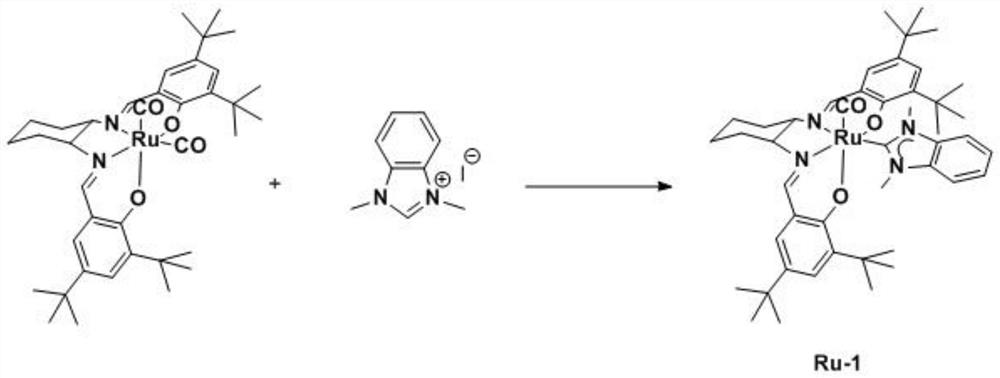
[0040] (2) Synthesis of cis-ruthenium-salen azacyclic carbene Ru-1
[0041] Under nitrogen protection, add cis-ruthenium-salen biscarbonyl complex precursor A (1mmol, 702mg), silver oxide (4mmol, 920mg), 1,3-dimethyl-1H-benzo Imidazolium-3-onium iodide (2mmol, 546mg), 100ml dehydrated tetrahydrofuran, after the addition of reactants, react at 50°C for 8h, then cool down to room temperature, remove the solvent by rotary evaporation, and separate and purify through silica gel chromatography to obtain an orange powder compound Ru-1, the yield was 79%.
[0042]
[0043] Ru-1: 1 H NMR (CDCl 3 ,300Hz)δ8.30(s,1H),7.78(s,1H),7.36(d,J=2.7Hz,1H),7.11(br,4H),7.02(d,J=2.6Hz,1...
Embodiment 2
[0045] The synthesis steps of the cis-ruthenium-salen azacyclic carbene Ru-4 of the present embodiment are as follows:
[0046] (1) Synthesis of Precursor B
[0047] Precursor B was synthesized according to the method disclosed in J.Am.Chem.Soc.2009, 131, 4405-4417, and the yield was 41%.
[0048]
[0049] B: 1 H NMR (CDCl 3 ,400Hz)δ8.08(s,1H),7.52(s,1H),7.39-7.34(m,3H),7.27-7.24(m,1H),7.16-7.15(m,2H),7.11(d, J=2.6Hz, 1H), 6.88(d, J=7.7Hz, 1H), 6.85(d, J=2.7Hz, 1H), 6.77-6.75(m, 1H), 2.15(s, 3H), 1.92( s,3H);IR 2065,1992cm -1 (υ(CO)); FAB-MSm / z 713[M] +
[0050] (2) Synthesis of cis-ruthenium-salen azacyclic carbene Ru-4
[0051]Under the protection of nitrogen, add the precursor Bcis-ruthenium-salen biscarbonyl complex (1mmol, 713mg), silver oxide (4mmol, 920mg), 1-ethyl-3-methylimidazolium iodide into a 100mL three-necked flask (2mmol, 476mg), 100ml dehydrated tetrahydrofuran, after the addition of the reactants is completed, react at 50°C for 8h, then cool down t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com