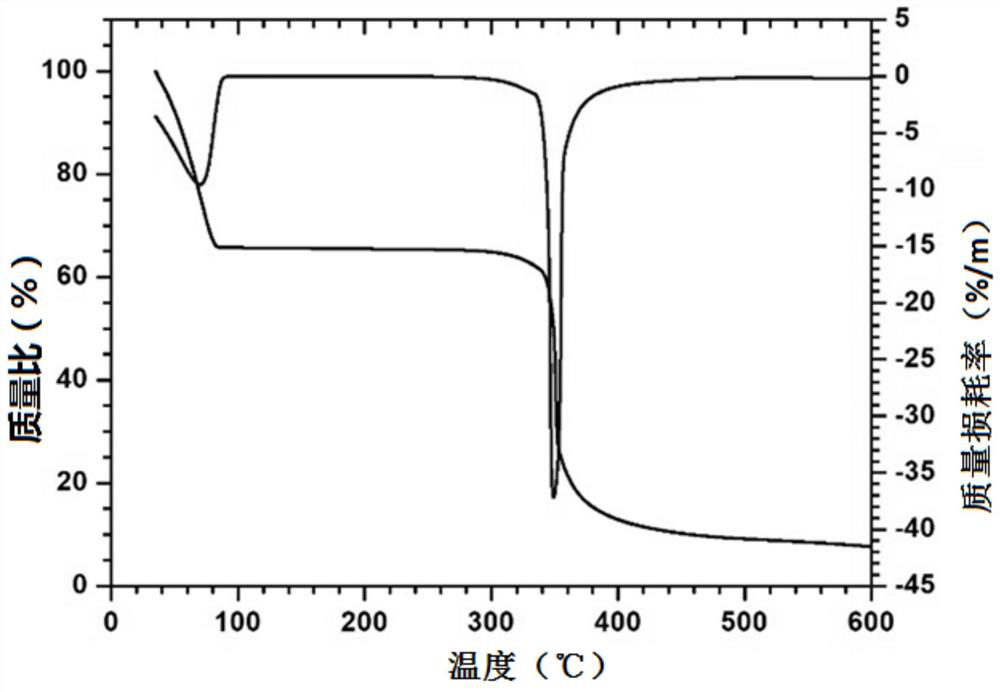
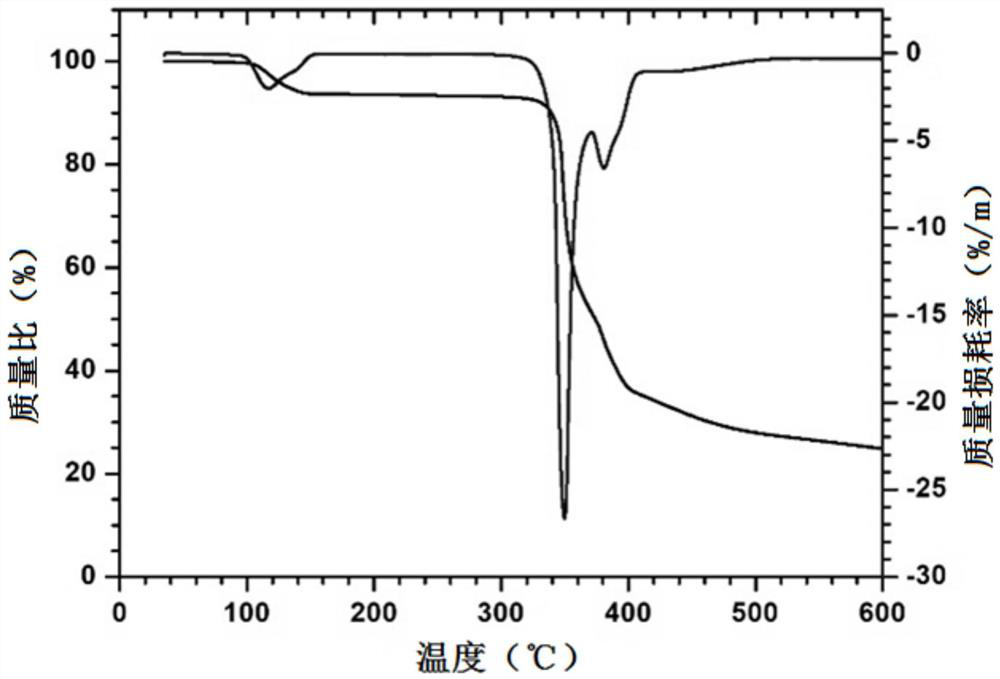
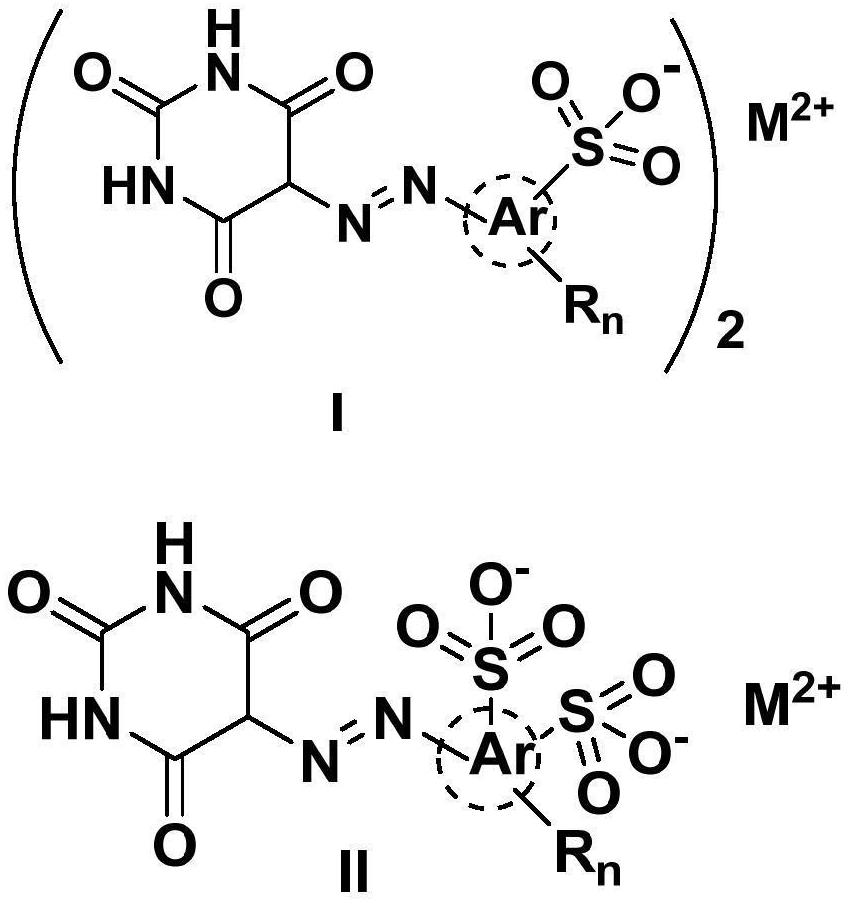
Monoazo lake pigment and preparation method thereof
A technology of lake pigments and monoazo, applied in the directions of monoazo dyes, azo dyes, lakes, etc., can solve the problem of no economical method, and achieve the effect of reducing residual amount, reducing treatment difficulty and economic cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The first step, diazotization reaction of aromatic primary amine sulfonic acid
[0046] Mix p-aminobenzenesulfonic acid (industrial product, Hebei Jianxin Chemical Co., Ltd., 17.4g, 0.1mol) with water (800ml), stir until the solids are completely dissolved, and obtain an aqueous solution of p-aminobenzenesulfonic acid (pH=1-2 ), cooled with an ice / water bath to reduce the solution temperature to <10°C. Under stirring, slowly add 40% nitrosylsulfuric acid (industrial product, 40% sulfuric acid solution, Zhejiang Sanmen Jieshi Chemical Co., Ltd., 33g, 0.105mol) into the above solution for about 30 minutes, during which the temperature is controlled <10°C. After the addition, continue to stir for 20 minutes to obtain a cloudy water solution of diazonium p-aminobenzenesulfonic acid, pH = 1, and the starch-potassium iodide test paper detects that there is a slight excess of nitrous acid.
[0047] The second step, the preparation of barbituric acid coupling solution
[0048...
Embodiment 2
[0056] The first step to the third step are the same as in Example 1.
[0057] The fourth step, the laking reaction
[0058] After the third step of the reaction, directly heat the reactant to 60°C and keep it warm for 1 hour to obtain a transparent and clear solution, adjust the pH to 5-6 with 10% KOH, and add CaCl dropwise 2 (Industrial product, 11.3g, 0.1mol) aqueous solution, after the dropwise addition, stop heating, cool naturally, and let stand overnight. The precipitated filter cake was filtered, washed with water until neutral, and dried at 120°C to obtain 33.2 g of a brilliant bright yellow solid, which was monoazo lake pigment I-1-1.
Embodiment 3
[0060] The first step, diazotization reaction of aromatic primary amine sulfonic acid
[0061] Mix Turmeric's Acid (industrial product, Inner Mongolia Xinya Chemical Co., Ltd., 22.7g, 0.1mol) and water (600ml), heat to 50°C under stirring, add 10% KOH solution dropwise until the solids are completely dissolved, and get Turmeric's Acid The aqueous solution of potassium salt is naturally cooled to room temperature, and the pH is adjusted to 1-2 with sulfuric acid. Turmeric acid is precipitated from the water to form a turbid solution. Use an ice / water bath to reduce the temperature of the turbid solution to <10°C. Under stirring, slowly add 40% nitrosylsulfuric acid (industrial product, 40% sulfuric acid solution, 33g, 0.105mol) into the above solution dropwise for about 30 minutes, during which the temperature is controlled to <10°C. After the addition, continue stirring for 20 minutes to obtain The diazonium salt of the acid (cloudy liquid, light yellow), pH = 1, starch-potass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com