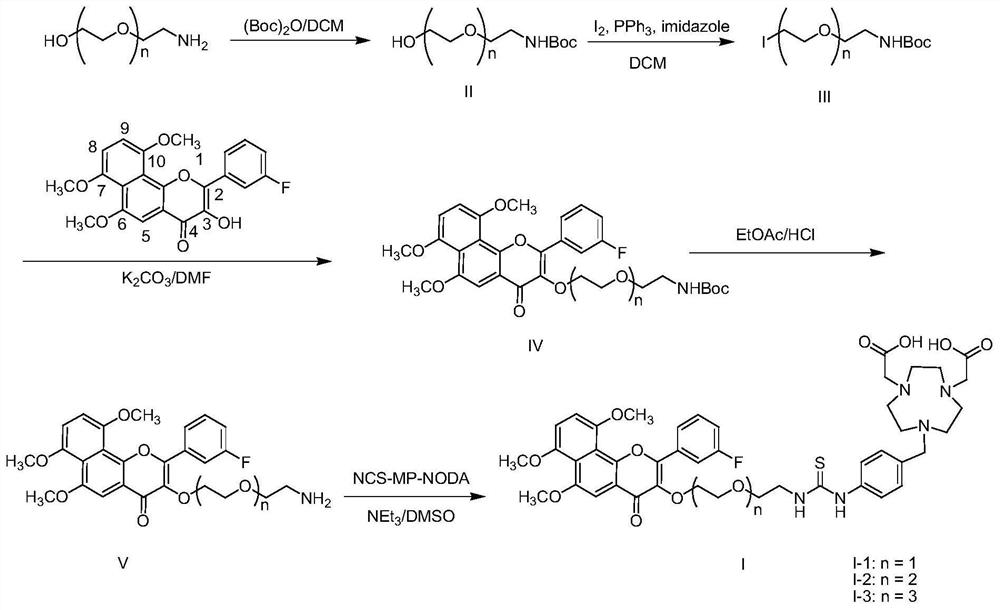
CYP1B1 enzyme targeting probe precursor for radioactivity <18> F labeling
A radioactive and labeling technology, applied in the field of PET imaging and tumor diagnosis, can solve the problems of low diagnostic specificity and difficult diagnosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The present embodiment relates to a kind of radioactive compound derived from 6,7,10-trimethoxy-3'-fluoro-α-naphthalene flavonol with structural formula I 18 Preparation method of F-labeled probe precursor I-3, such as figure 1 shown, including the following steps:
[0029] Step 1: Dissolve 2-(2-(2-(2-aminoethoxy)ethoxy)ethoxy)ethanol (2mmol) in 6mL of dichloromethane, add dropwise in 4mL dichloromethane under ice-cooling Di-tert-butyl dicarbonyl ester of methyl chloride (2.3 mmol). After the dropwise addition, the ice bath was removed, and the reaction solution was stirred overnight at room temperature. After the reaction, the reaction solution was diluted with 10 mL of dichloromethane, and the organic phase was washed successively with an equal volume of water, saturated sodium bicarbonate solution and saturated sodium chloride solution. After drying the organic phase with anhydrous sodium sulfate, it was concentrated under reduced pressure to obtain a colorless oi...
Embodiment 2
[0035] The present embodiment relates to a kind of radioactive compound derived from 6,7,10-trimethoxy-3'-fluoro-α-naphthalene flavonol with structural formula I 18 The preparation method of F-labeled probe precursor I-1, such as figure 1 shown, including the following steps:
[0036] Step 1: Same as Step 5 of Example 1, replace V-3 with V-1 (see published paper J.Med.Chem., 2018, 61, 10901-10909 for the synthesis method), Rf=27.525min, and obtain an orange-yellow solid I-1 (n=1). Yield, 52.4%. 1 H NMR (600MHz, DMSO-d 6 ):δ9.80(s,1H),8.27-8.35(m,2H),7.67-7.70(m,1H),7.58(d,J=6.0Hz,2H),7.41-7.44(m,3H), 7.30-7.32(m,3H),4.41(s,2H),4.28(s,2H),4.06(s,3H),3.94(s,3H),3.84(s,3H),3.75(s,2H) ,3.64(s,2H),3.55-3.57(m,2H),3.42-3.47(m,4H),3.30(m,2H),3.11(m,4H),3.00-3.03(m,2H),2.79 (m,2H),2.61-2.68(m,4H). 13 C NMR (150MHz, DMSO-d 6 ): δ181.09, 173.26, 173.12, 162.52(d, J=207.0Hz), 154.57, 152.61, 151.26, 151.05, 147.83, 140.92, 133.60(d, J=7.5Hz), 131.09(d, J=6.0Hz), 130.87, 125.00, ...
Embodiment 3
[0038] The present embodiment relates to a kind of radioactive compound derived from 6,7,10-trimethoxy-3'-fluoro-α-naphthalene flavonol with structural formula I 18 Preparation method of F-labeled probe precursor I-2, such as figure 1 shown, including the following steps:
[0039] step one:
[0040] Same as Step 5 of Example 1, replace V-3 with V-2 (see the published paper J.Med.Chem., 2018, 61, 10901-10909 for the synthesis method, Rf=27.865min, and obtain orange-yellow solid I-2 (n=2), yield: 72%. 1 H NMR (600MHz, DMSO-d 6 ):δ9.77(s,1H),8.28-8.37(m,2H),7.67-7.71(m,1H),7.57(d,J=6.6Hz,2H),7.43-7.49(m,3H), 7.31-7.33(m,3H),4.40(m,2H),4.29(s,2H),4.07(s,3H),3.95(s,3H),3.85(s,3H),3.72(m,2H) ,3.62(m,2H),3.53-3.54(m,6H),3.42-3.48(m,4H),3.30(m,2H),3.12(m,4H),3.01-3.03(m,2H),2.79 (m,2H),2.61-2.68(m,4H). 13 C NMR (150MHz, DMSO-d 6 ): δ181.03, 173.25, 173.14, 162.52(d, J=207.0Hz), 154.57, 152.57, 151.28, 151.08, 147.82, 140.90, 133.62(d, J=7.5Hz), 131.09(d, J=6.8Hz), 130.87, 125...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com