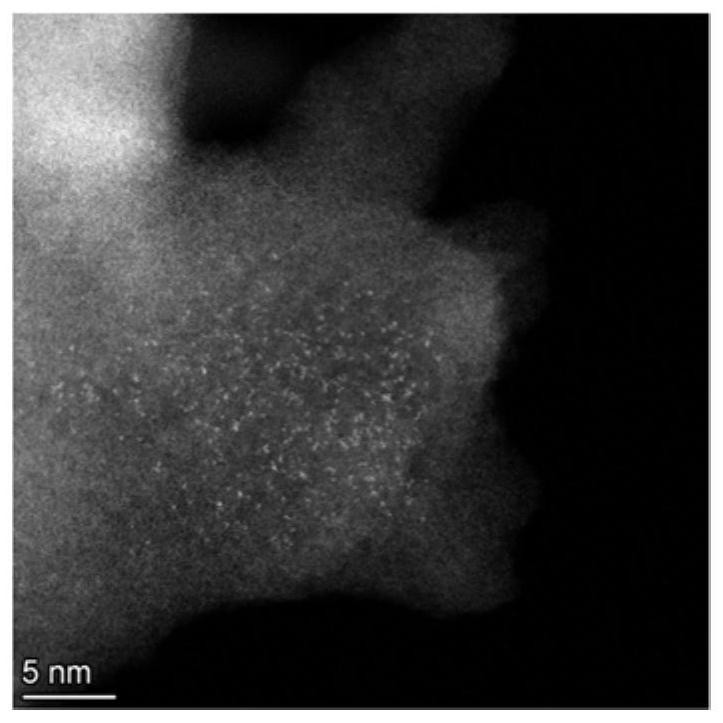
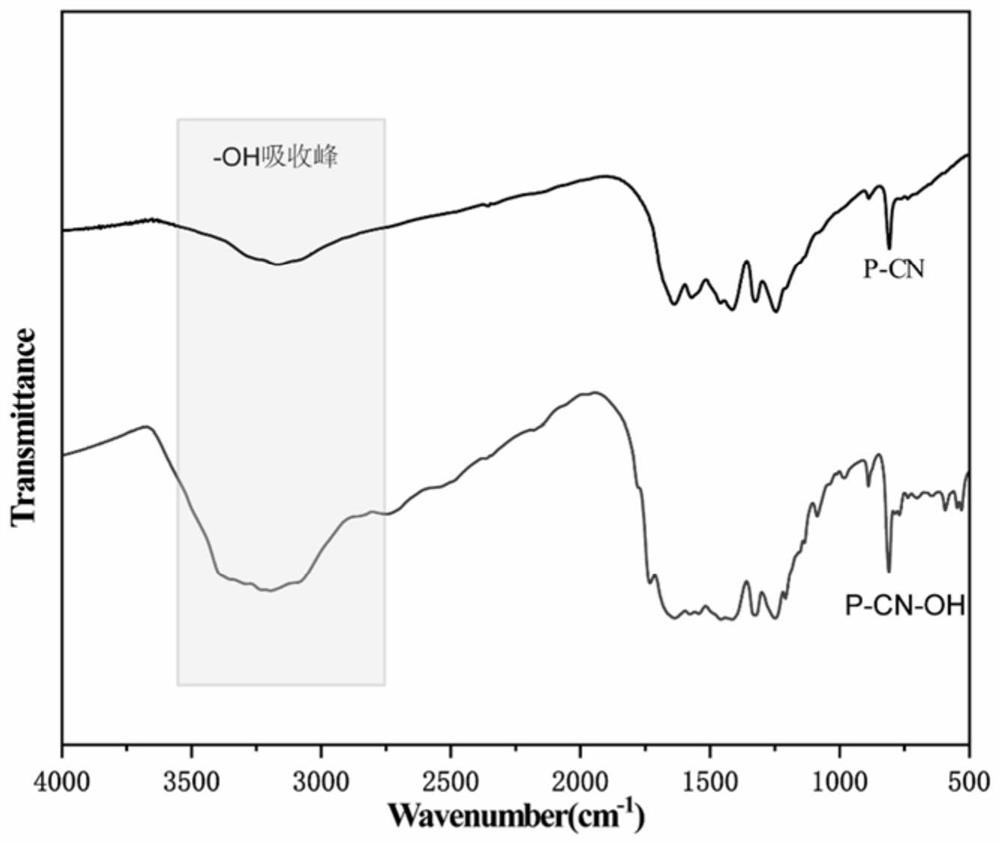
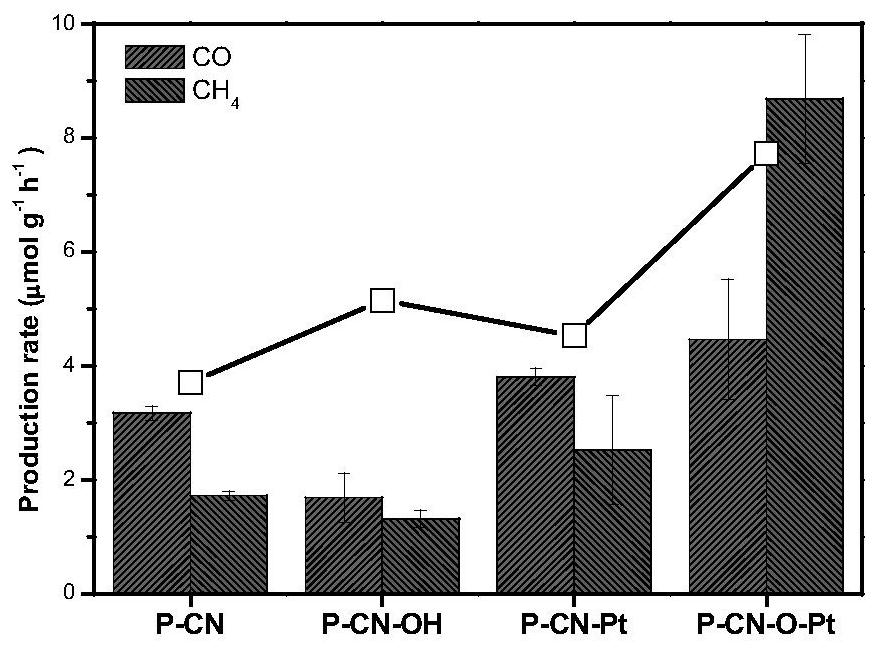
Metal single atom loaded carbon-nitrogen polymer catalyst and preparation method thereof
A technology for polymers and catalysts, which is applied in the field of metal single-atom-supported carbon-nitrogen polymer catalysts and its preparation. The effect of large amount and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The preparation method of the metal single atom supported carbon nitrogen polymer catalyst provided by the invention comprises the following steps:
[0036] (1) Put 1-10g of nitrogen-containing precursor into a 10-100mL alumina crucible with a cover, then put it into a muffle furnace, and raise the temperature from room temperature to 520-580°C at a rate of 1-5°C, And calcined at this temperature for 2-5h.
[0037] Wherein, the nitrogen-containing precursor is one or more of urea, melamine and cyanuric acid, when two of urea, melamine and cyanuric acid are used, the two are mixed according to the mass ratio of 1:1 , when three kinds are used, the three kinds are mixed according to the mass ratio of 1:1:1.
[0038] (2) Grinding the block solid obtained after calcination to below 200 mesh to obtain a carbon nitrogen polymer, which is denoted as P-CN.
[0039] (3) Take 0.1-0.5g P-CN and add it to a 30-100mL reaction kettle, add 20-70mL deionized water, and conduct a hydr...
Embodiment 1
[0057] (1) Put 5g of melamine into a 100mL alumina crucible with a cover, and calcinate in a muffle furnace for 3h, with a heating rate of 5°C and a calcining temperature of 550°C.
[0058] (2) Grinding the massive solid obtained after the former is calcined to below 200 mesh to obtain P-CN.
[0059] (3) Take 0.3g of the P-CN prepared by the former and add it to a 100mL reactor, add 70mL of deionized water, and conduct a hydrothermal treatment in an oven at 180°C for 12 hours. After the reaction is completed, the solution is centrifuged, and absolute ethanol and deionized water are alternately Wash 3 times, dry at 80°C for 12 hours, and grind to below 200 mesh to obtain a carbon-nitrogen polymer containing a large number of hydroxyl groups, which is denoted as P-CN-OH.
[0060] (4) Put 0.3g of the P-CN-OH prepared by the former into a 200mL beaker, add 40mL of deionized water, and ultrasonically treat it with a power of 200W for 30min to disperse the P-CN-OH evenly in water. ...
Embodiment 2
[0070] (1) Put 5g of melamine into a 100mL alumina crucible with a cover, and calcinate in a muffle furnace for 3h, with a heating rate of 5°C and a calcining temperature of 550°C.
[0071] (2) Grinding the massive solid obtained after the former is calcined to below 200 mesh to obtain P-CN.
[0072] Take 0.3g of P-CN and add it to a 100mL reactor, add 70mL of deionized water, and conduct a hydrothermal treatment in an oven at 180°C for 12 hours. Dry at ℃ for 12 hours, and grind to below 200 mesh to obtain a carbon-nitrogen polymer containing a large number of hydroxyl groups, which is recorded as P-CN-OH.
[0073] (3) Put 0.3g of P-CN-OH into a 200mL beaker, add 40mL of deionized water, and sonicate with a power of 200W for 30min to disperse P-CN-OH in water evenly.
[0074] (4) Add 10mL of 0.1mol / L CuCl to the solution obtained from the former 2 The solution was stirred at 500 r / min at room temperature and protected from light for 10 hours. After the stirring was completed...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com