Preparation method of imatine
An imaamine and enzymatic technology, applied in the field of preparation of imaamine, can solve the problems of low product yield, long reaction route and high cost, and achieve the effects of high product conversion rate and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
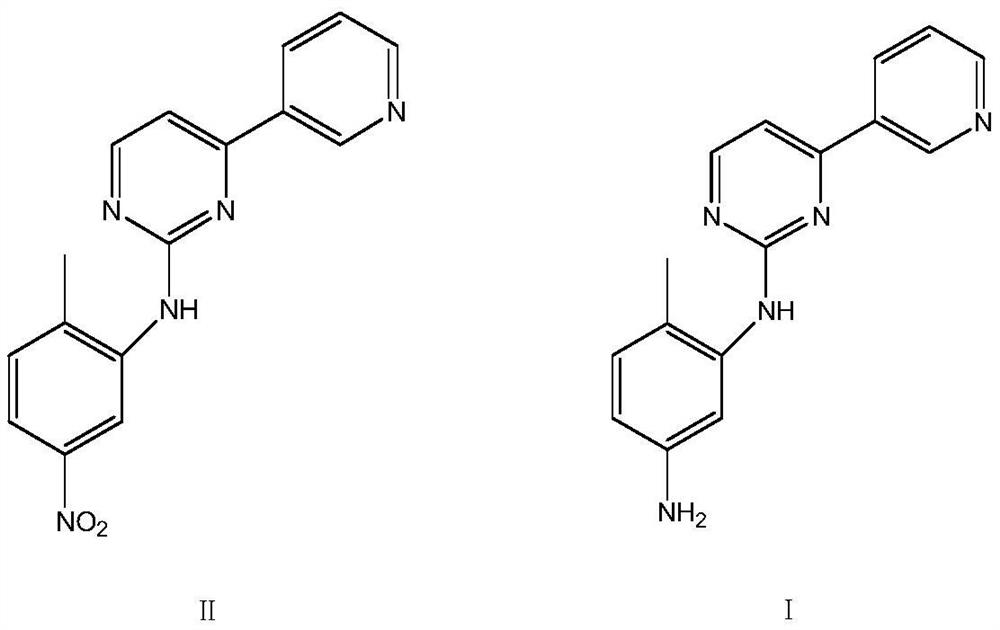
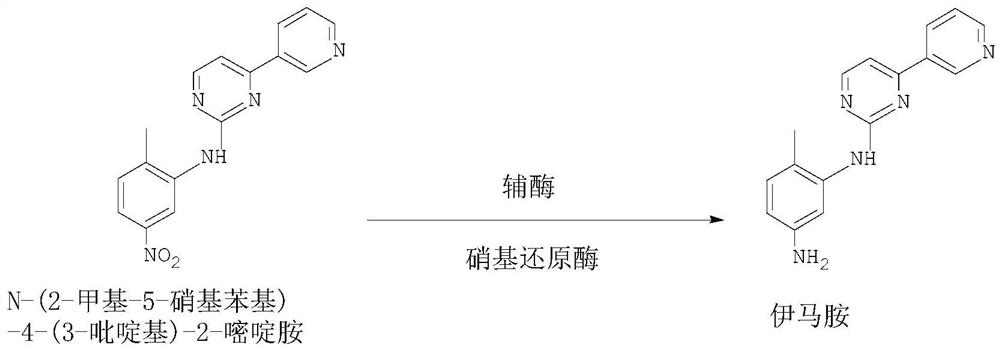
[0020] In the clean reactor, add water 100g, ethanol 20g, then add raw material N-(2-methyl-5-nitrophenyl)-4-(3-pyridyl)-2-pyrimidinamine 50g, adopt SEQ ID NO 1. Add 2 g of nitroreductase and 0.2 g of coenzyme, stir, slowly raise the temperature to 37 ° C for 2 hours of reaction, after the reaction, collect the filtrate after suction filtration, perform vacuum distillation to remove solvent ethanol, suction filtration, and dry Dry to obtain the corresponding product 44.1g imamamine, the yield is 97.8%, and the liquid phase (HPLC) detection content is 99.5%.
Embodiment 2
[0022] Add water 100g, ethanol 20g, N-(2-methyl-5-nitrophenyl)-4-(3-pyridyl)-2-pyrimidinamine 50g successively in clean reactor, adopt SEQ ID NO 1 2g of the shown nitroreductase, 0.2g of coenzyme, stirring, slowly heating up to about 35°C for reaction and heat preservation for 2h, suction filtration, distillation to remove solvent ethanol, suction filtration, drying to obtain 44.0g of the corresponding product imamamine, The yield is 97.6%, and the liquid phase (HPLC) detection content is 99.6%.
Embodiment 3
[0024] Add water 100g, methanol 20g, N-(2-methyl-5-nitrophenyl)-4-(3-pyridyl)-2-pyrimidinamine 50g successively in clean reactor, adopt SEQ ID NO 1 .The nitroreductase 2g shown, the coenzyme 0.2g, the temperature is raised to about 37 ℃, the reaction is incubated for 2h, the solvent ethanol is distilled off by suction filtration, the solvent ethanol is distilled off, the corresponding product 44.3g imamamine is obtained, and the yield is 98.2 %, liquid phase (HPLC) detection content 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com