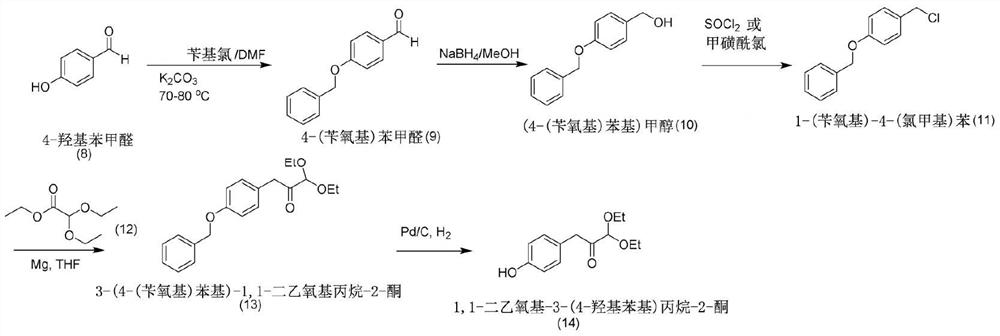
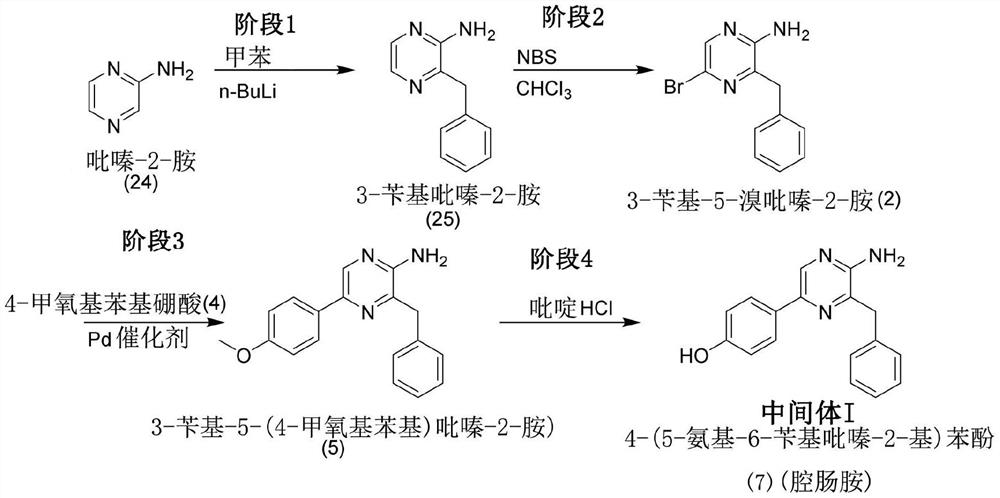
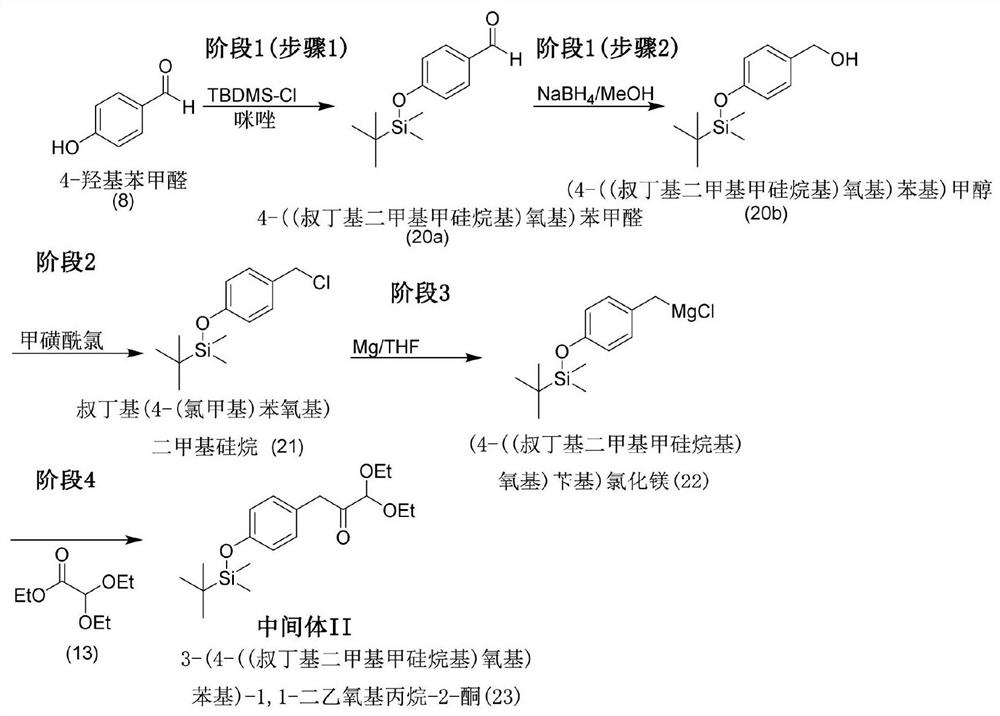
Synthesis of coelenterazine
A technology for preparing cavities and benzyl groups, which is applied in the public domain and can solve problems such as reducing the practicality of the indication mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0174] Embodiment 1. the synthesis of coelenterazine (1 kilogram)
[0175] Synthesis of 3-Benzylpyrazin-2-amine (25)
[0176]
[0177] 10 L of toluene was added to a 100 L round bottom flask, followed by 10 L of tetramethylethylenedienylamine (TMEDA). The mixture was cooled to 0-5°C with ice water. Under nitrogen, at 1 to 1 1 / 2 To the stirred mixture was added dropwise 20 L of a solution of n-butyllithium (2.5M in n-hexane) at 0°C to 8°C over a period of hours. After the addition was complete, the reaction mixture was allowed to warm to room temperature (22°C). After standing at room temperature for 20 minutes, the mixture was slowly heated to 60° C. (butane gas was evolved during this time). The reaction mixture was maintained at 60°C ± 1°C for 30 minutes and then cooled to room temperature.
[0178] Simultaneously, tetrahydrofuran (THF) (15 L) and pyrazin-2-amine (24) (also known as 2-aminopyrazine (24)) (1 Kg) were added to another 30 L flask. The mixture wa...
Embodiment 2
[0204] Embodiment 2: the synthesis of coelenterazine (16)
[0205] 3-Benzyl-5-bromopyrazin-2-amine (2)
[0206]
[0207] Under an argon atmosphere, zinc powder and 30 mesh granular zinc (1:1, 16.05 / 16.05g, 491.2mmol, 3.5 equivalents) and I 2 (6.23 g, 5% of Zn) was added to a dry 1 L two necked round bottom flask. Add N,N'-dimethylacetamide (125mL, freshly treated with CaH 2 distillation). The mixture was stirred at room temperature until I 2 Brown color disappears. Benzyl bromide (61.04 g, 356.9 mmol, 2.5 equiv) was added dropwise, and the mixture was stirred at 80° C. for 4 hours. The mixture was cooled to room temperature, and 3,5-dibromo-2-aminopyrazine (1) (36.0 g, 140.3 mmol, 1 equiv) and PdCl were added 2 (PPh 3 ) 2 (5.04 g, 0.712 mmol, 5% of pyrazine) in N,N'-dimethylacetamide (150 mL). The mixture was continuously stirred under argon atmosphere for 1 day. Thin layer chromatography (TLC) (30% ethyl acetate / hexanes) showed the reaction was complete. The...
Embodiment 3
[0256] Example 3: Synthesis of coelenterazine hydrochloride, 100 gram scale
[0257] Starting material synthesis:
[0258] Starting materials 4-(5-amino-6-benzyl-pyrazin-2-yl)phenol (7) (53.0 g) and 1,1-diethoxy-3-(4-hydroxyphenyl)propane -2-Kone (14) (57.0 g) was synthesized by following the procedure previously described in Example 2.
[0259] 8-Benzyl-6-(4-hydroxyphenyl)-2-[(4-hydroxyphenyl)methyl]-7H-imidazo[1,2-a]pyrazin-3-one (3)
[0260]
[0261] In a 1L round bottom flask equipped with a stir bar was added 4-(5-amino-6-benzyl-pyrazin-2-yl)phenol (7) (53.0 g, 191.25 mmol) followed by 1,4-di Oxane (275 mL). The resulting mixture was degassed and filled with argon. To the stirred mixture was added degassed 1:1 water / cone HCl (62.0 mL). The resulting mixture was again degassed and filled with argon and stirred at room temperature for 15 minutes. To this stirred suspension was added 1,1-diethoxy-3-(4-hydroxyphenyl)-2-propan-2-one (14) in degassed 1,4-dioxane (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com