Severe acute respiratory syndrome coronavirus 2 affinity peptide based on human angiotensin-converting enzyme 2
A technology of angiotensin and respiratory system, which is applied in the field of biomaterials, can solve the problems of the length of the peptide chain limiting the scope of use and the inability to cover key sites, etc., and achieves good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1 SARS-CoV-2 affinity polypeptide design based on hACE2
[0042] 1. Recognition and remodeling of key action sites of hACE2 and SARS-COV-2S_RBD complex
[0043] The co-crystal structure of hACE2 and SARS-COV-2S_RBD complex was used as a reference for analysis and the principle of peptide inhibition.
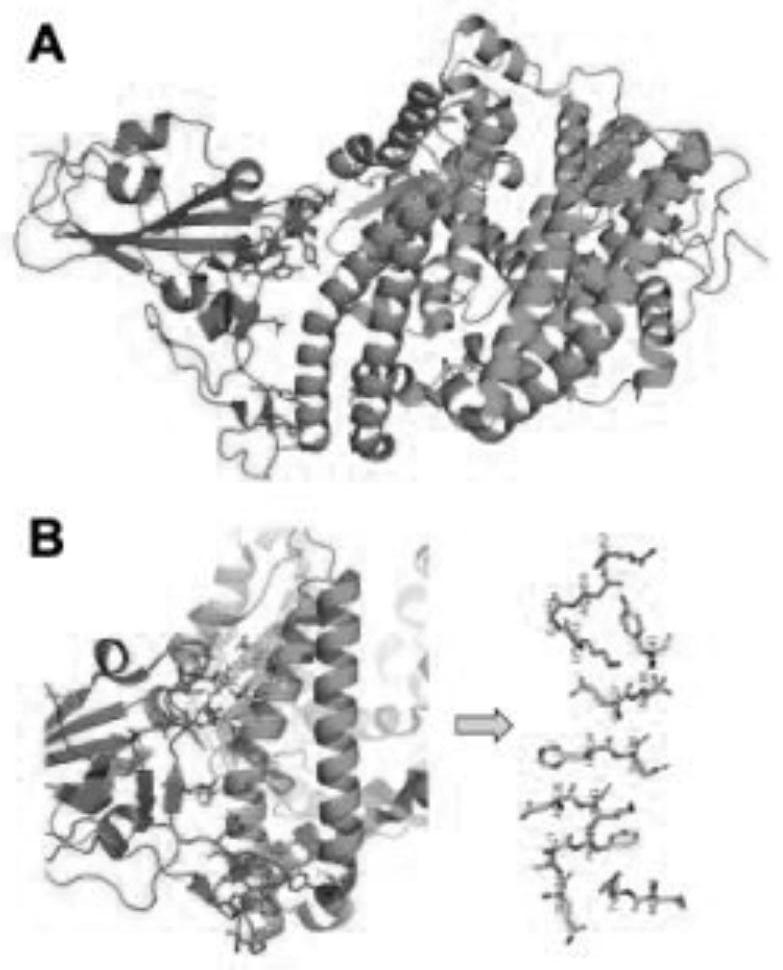
[0044] figure 1 Shown is the crystal structure showing the co-crystallization of hACE2 and SARS-COV-2S_RBD complex. The crystal structure of the co-crystal of hACE2 and SARS-COV-2S_RBD complex was determined by X-ray crystallography analysis. Both hACE2 and SARS-COV-2 interact through the protein surface to form a complex. If the specific information on the binding surface of hACE2 and SARS-COV-2 is clear, specific polypeptides can be designed to compete for binding to the surface of SARS-COV-2, thereby preventing the binding of hACE2 and achieving the purpose of inhibiting SARS-COV-2.
[0045] figure 1 (A) S_RBD is shown in green, hACE2 is shown in blue. ...
Embodiment 2
[0074] Example 2 SARS-COV-2S_RBD Rapid Biodetection Method Based on Affinity Peptide Design
[0075] According to the SARS-CoV-2S_RBD affinity polypeptide obtained in Example 1, the affinity polypeptide Lib15-2 and Lib12-1 with non-overlapping binding sites were selected as biorecognition materials, and the method for rapid biological detection of SARS-CoV-2S_RBD was designed .
[0076] The affinity polypeptide Lib15-2 and Lib12-1 were respectively treated with -CONH-(CH 2 ) 7 -Cys modification, the affinity peptides Lib15-2 and Lib12-1 were modified onto gold nanoparticles (AuNPs) by a co-bonding method to obtain two functional biosensing materials, AuNPs probes (peptide-AuNPs), and the above Two peptide-functionalized modified gold nanoparticles were mixed in a 1:1 ratio to form a biodetection reagent. The specific steps include: 1) using the polypeptide solid-phase synthesis method to synthesize the polypeptide, and inserting the linker 8-aminocaprylic acid and cysteine ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com