Lyophilized preparation of metabolic modulatory fusion protein
A technology of freeze-dried preparations and fusion proteins, which is applied in the field of freeze-dried preparations for metabolically regulated fusion proteins, which can solve the problems of easy aggregation and instability of proteins, and achieve the effects of rapid reconstitution, good drying effect, and uniform texture
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
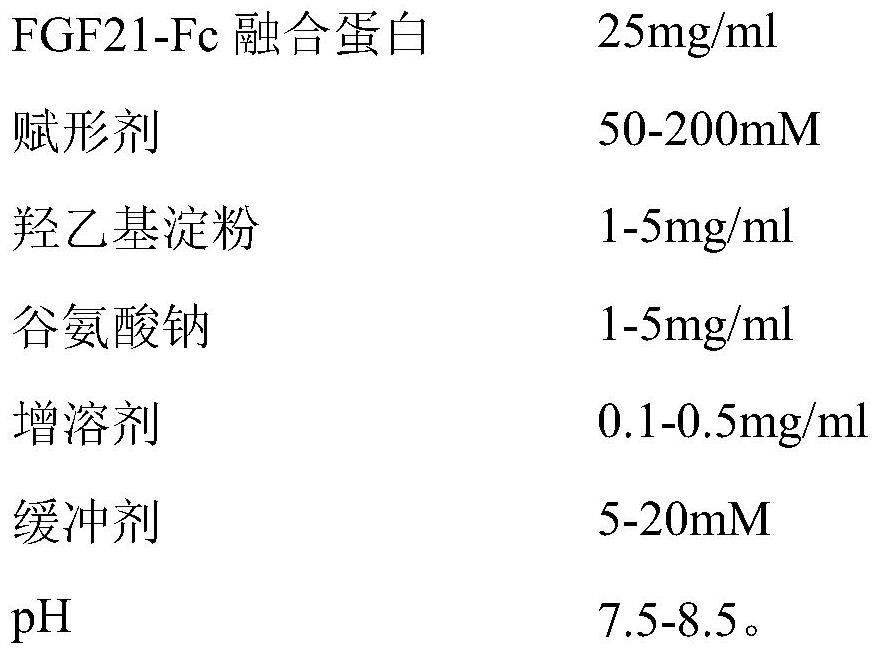
[0036] (1) Prescription
[0037]
[0038] (2) Preparation method
[0039] Weigh the prescribed amount of Tris, dissolve it with an appropriate amount of water for injection, adjust the pH with hydrochloric acid, take a sample to test the pH and endotoxin, and then accurately weigh the prescribed amount of maltose, hydroxyethyl starch, sodium glutamate, Tween 80 and recombinant Add the FGF21-Fc fusion protein stock solution into Tris / HCl buffer solution, mix evenly to get the semi-finished solution, filter the semi-finished solution with a 0.22μm filter membrane, and fill it into a vial after passing the endotoxin test. 7ml vial, the filling volume is 1ml.
[0040] (3) Freeze-drying process
[0041] 1) Pre-freezing: first place the vial containing the recombinant FGF21-Fc fusion protein solution on the partition plate of the freeze dryer, set the temperature for pre-freezing at 2°C, set the time for 10 minutes to reach the preset temperature, maintain it for 30 minutes, and ...
Embodiment 2
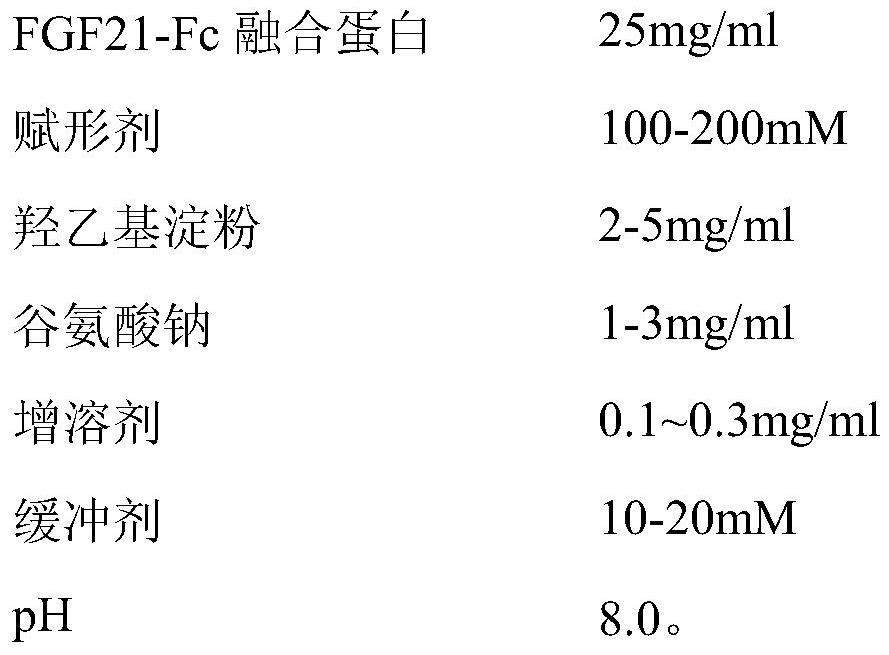
[0045] (1) Prescription
[0046]
[0047]
[0048] The preparation method and freeze-drying process are the same as in Example 1.
Embodiment 3
[0050] (1) Prescription
[0051]
[0052] (2) Preparation method
[0053] Weigh the prescribed amount of Tris, dissolve it with an appropriate amount of water for injection, adjust the pH with hydrochloric acid, take a sample to test the pH and endotoxin, and then accurately weigh the prescribed amount of maltose, hydroxyethyl starch, sodium glutamate, Tween 80 and recombinant Add the FGF21-Fc fusion protein stock solution into Tris / HCl buffer solution, mix evenly to get the semi-finished solution, filter the semi-finished solution with a 0.22μm filter membrane, and fill it into a vial after passing the endotoxin test. 7ml vial, the filling volume is 1ml.
[0054] (3) Freeze-drying process
[0055] 1) Pre-freezing: first place the vial containing the recombinant FGF21-Fc fusion protein solution on the partition plate of the freeze dryer, set the temperature for pre-freezing at 2°C, set the time for 10 minutes to reach the preset temperature, maintain it for 30 minutes, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com