Therapeutic lung repair by inhalation of lung spheroid cell-secreted factors
A technology of cell secretion and spheres, which is applied in the direction of respiratory/lung cells, animal cells, vertebrate cells, etc., and can solve problems of labor, high cost, and large quantities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
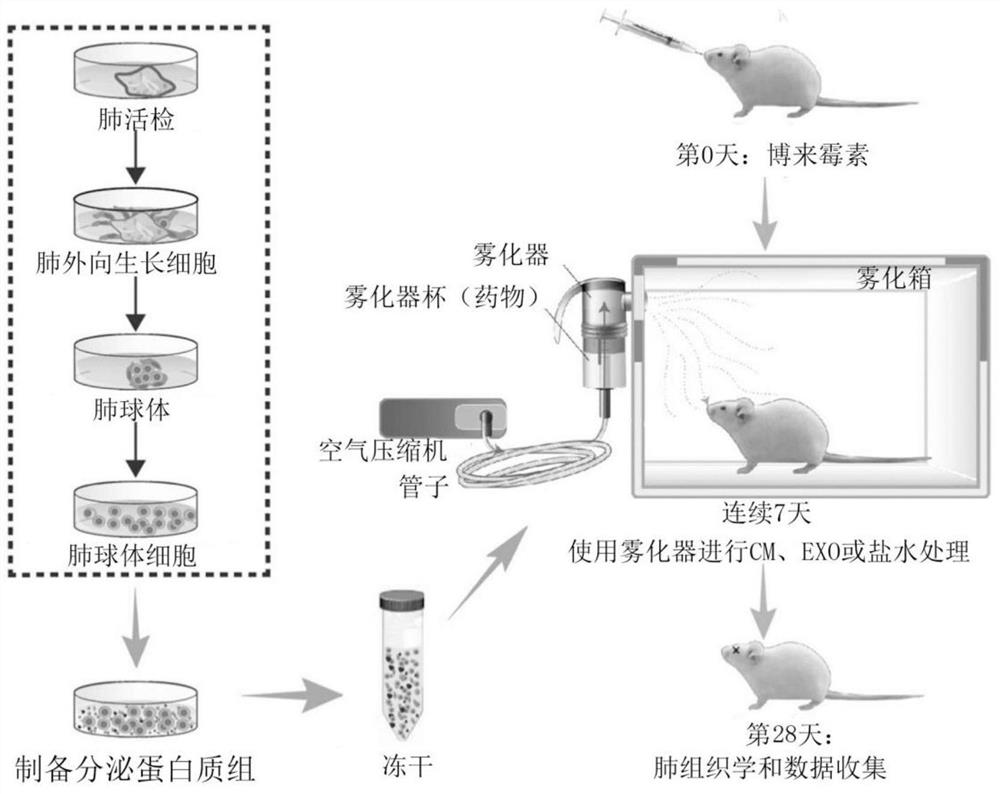
[0236] Cell Culture: Human LSCs were generated from whole lung samples and expanded as described by Dinh et al., (2017) RespiratoryResearch. Human LSCs were prepared from healthy whole lung donors and expanded as described in Figure 5. Passage 2-5 LSCs were used for all in vitro and in vivo experiments. Cells were also analyzed by flow cytometry for appropriate markers (CD90+, CD105+, CCSP+, AQP5+, ProSPC+, Epcam+, CD31-, CD34- and CD45-) before use.
Embodiment 2
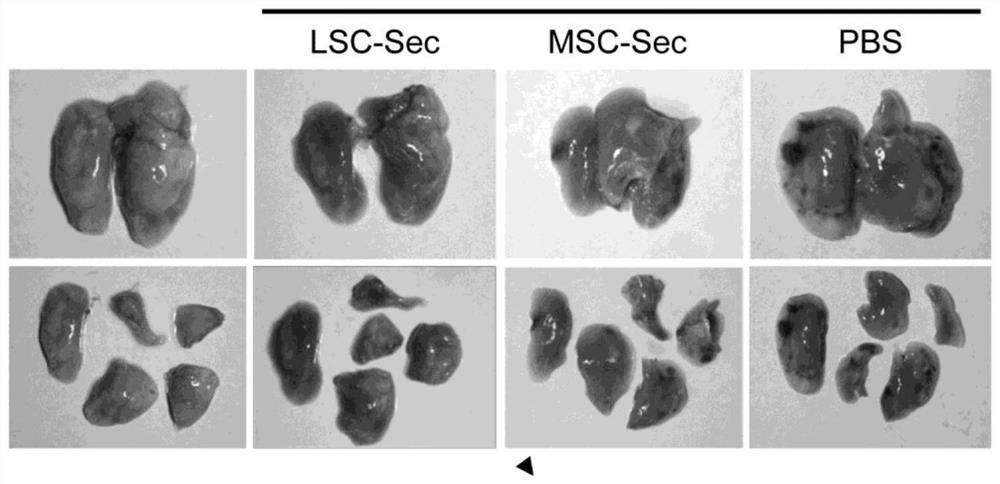
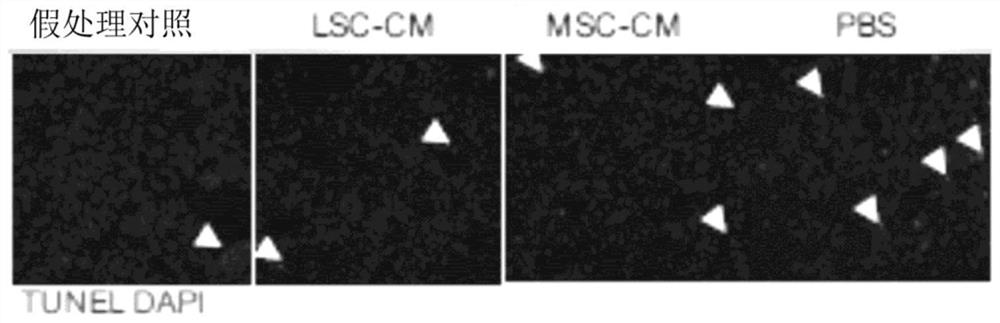
[0238] Collection and preparation of conditioned media: Human LSCs and MSCs were cultured to approximately 75% confluency before serum-containing media was removed and replaced with serum-free media (IMDM). The next day, cells were washed 6 times with fresh IMDM for 30 min each to deplete albumin on cells prior to media conditioning, as albumin interferes with some experiments (especially LC / MS / MS analyze). The medium (IMDM) was allowed to condition for three days before it was harvested and filtered through a 0.22 μm filter to remove any cells and cell debris. Store filtered CM at -80 °C for at least 24 h, or until solid. Freeze dry the frozen CM vials overnight or until the samples are dehydrated using the LABCONCO FreeZone 2.5 L Freeze Drying System. Once samples were lyophilized, they were stored at -20°C until ready to use.
Embodiment 3
[0240] Isolation and Characterization of Exosomes: Exosomes were collected and purified from human LSC-CMs by ultrafiltration. LSC-CMs are first filtered through a 0.22 μm filter to remove any cells or cell debris. The filtered medium was then placed on a 100 kDa MWCO ultrafiltration filter (Millipore) and centrifuged at 5000 RCF for 10-15 minutes depending on the volume. Any media content or small proteins were removed by filter centrifugation, and the remaining exosomes were suspended in PBS, then filtered and washed. Before use, all exosome samples were size-appropriately analyzed by nanoparticle tracking analysis (NTA; NanoSight, Malvern) and morphologically analyzed by transmission electron microscopy (TEM). Furthermore, successful exosome isolation was confirmed by immunoblotting for known exosomal markers (CD63 and CD81).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com