Polyselenoamino acid amphiphilic block copolymer as well as preparation method and application thereof
A technology of amphiphilic block and selenoamino acid, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of poor water solubility, degradation rate and cycle difficult to control, etc., to reduce the frequency of administration, reduce the Toxic and side effects, the effect of improving the therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
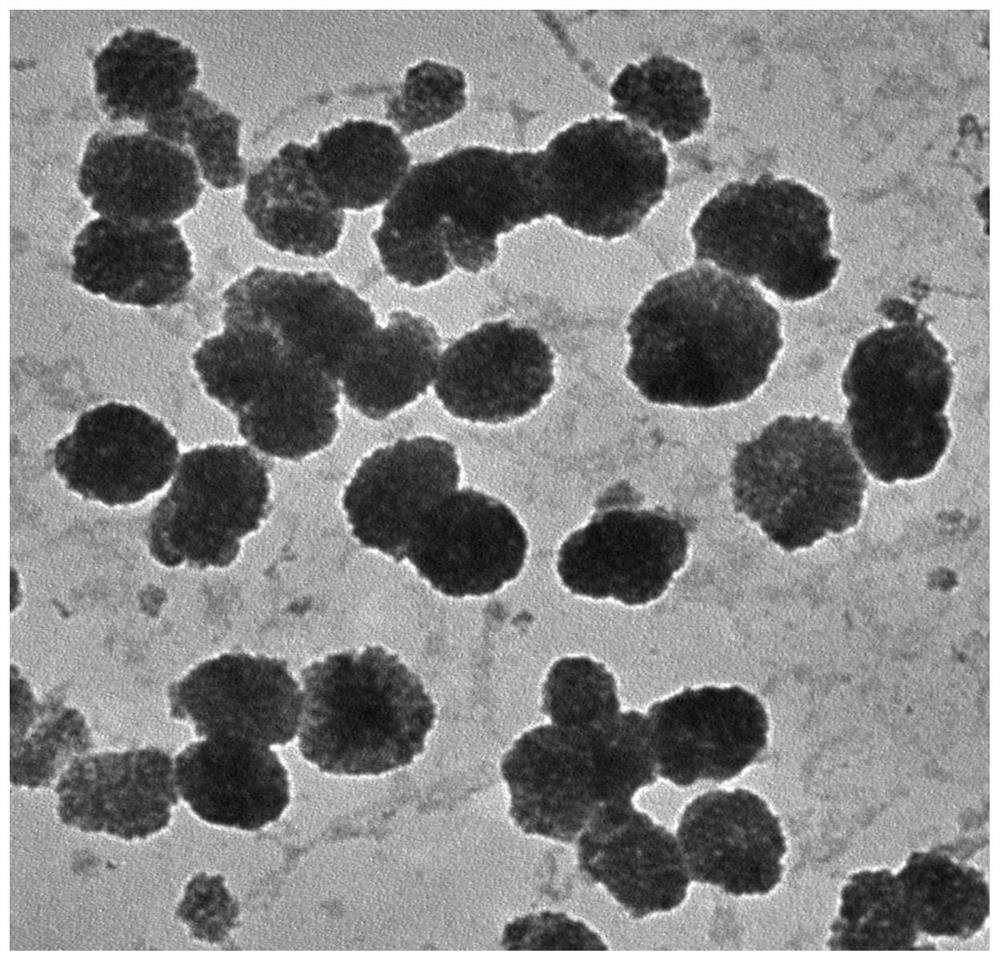
Image
Examples
Embodiment 1
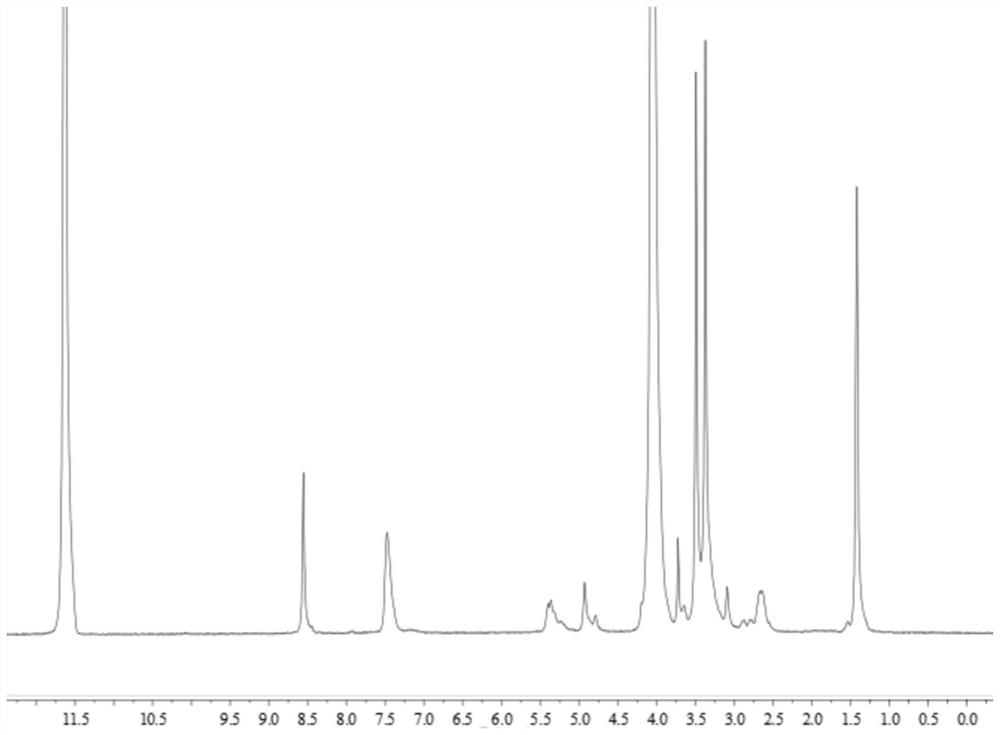
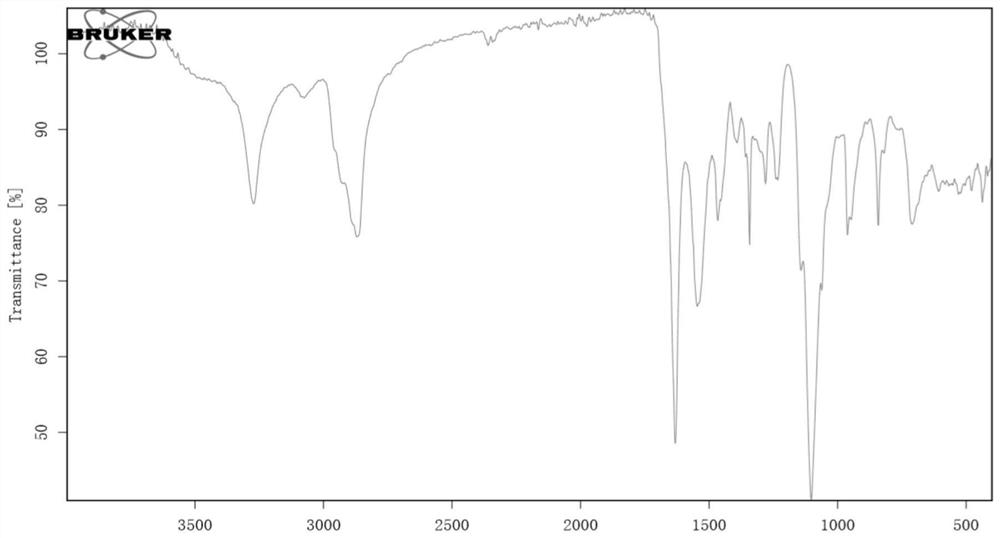
[0116] Polyethylene glycol monomethyl ether polyaspartic acid polyselenomethionine block copolymer (mPEG 45 -Pasp2-PMet(Se) 2 )Synthesis
[0117] Weigh 0.1g mPEG-NH 2 (0.05 mmol, Mn=2000) was vacuum-dried in a vacuum oven for 4 hours, and then dissolved in 3 ml of dried DMF to be used as a macroinitiator. Weigh 25mg of newly prepared benzyl L-aspartate NCA (BLA-NCA) (0.1mmol) and dissolve it in 1ml of ultra-dry DMF, then mix the two reaction solutions with a syringe, place under nitrogen atmosphere, and stir the reaction 24h. After the reaction, 22.3 mg of newly prepared seleno-L-methionine NCA (0.1 mmol) was weighed and dissolved in 1 ml of ultra-dry DMF, added to the above mixed reaction solution, and the reaction was continued with stirring. After 24 hours, the reaction was complete. The tri-block polymer was settled in ice anhydrous isopropyl ether, centrifuged, freeze-dried, and dried in a vacuum oven for 24 hours to obtain the product polyethylene glycol monomethyl ...
Embodiment 2
[0119] Polyethylene glycol monomethyl ether polyaspartic acid polyselenomethionine block copolymer (mPEG 45 -PAsp 2 -PMet(Se) 4 )Synthesis
[0120] Weigh 0.1g mPEG-NH 2(0.05 mmol, Mn=2000) was vacuum-dried in a vacuum oven for 4 hours, and then dissolved in 3 ml of dried DMF to be used as a macroinitiator. Weigh 25 mg of newly prepared L-benzyl-aspartate NCA (0.1 mmol) and dissolve it in 1 ml of ultra-dry DMF, then mix the two reaction solutions with a syringe, place under nitrogen atmosphere, and stir for 24 h. After the reaction, 44.6 mg of newly prepared seleno-L-methionine NCA (0.2 mmol) was weighed and dissolved in 1 ml of ultra-dry DMF, added to the above mixed reaction solution, and the reaction was continued with stirring. After 24 hours, the reaction was complete. The tri-block polymer was settled in ice anhydrous isopropyl ether, centrifuged, freeze-dried, and dried in a vacuum oven for 24 hours to obtain the product polyethylene glycol monomethyl ether polyaspa...
Embodiment 3
[0122] Polyethylene glycol monomethyl ether polyaspartic acid polyselenomethionine block copolymer (mPEG 45 -PAsp 2 -PMet(Se) 6 )Synthesis
[0123] Weigh 0.1g mPEG-NH 2 (0.05 mmol, Mn=2000) was vacuum-dried in a vacuum oven for 4 hours, and then dissolved in 3 ml of dried DMF to be used as a macroinitiator. Weigh 25 mg of newly prepared L-benzyl-aspartate NCA (0.1 mmol) and dissolve it in 1 ml of ultra-dry DMF, then mix the two reaction solutions with a syringe, place under nitrogen atmosphere, and stir for 24 h. After the reaction, weigh 66.9g of newly prepared seleno-L-methionine NCA (0.3mmol) and dissolve it in 1ml of ultra-dry DMF, add it to the above mixed reaction solution, and continue to stir the reaction. After 24 hours, the reaction was complete. The tri-block polymer was settled in ice anhydrous isopropyl ether, centrifuged, freeze-dried, and dried in a vacuum oven for 24 hours to obtain the product polyethylene glycol monomethyl ether polyaspartate benzyl este...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com