Two kinds of anti-her2 antibody-chaetokin conjugates and their preparation method and anti-tumor application
A technology of chaetin and chaetin, which is applied in the field of biotechnology and medicine, to reduce toxic and side effects, have great therapeutic application value, and enhance the effect of killing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
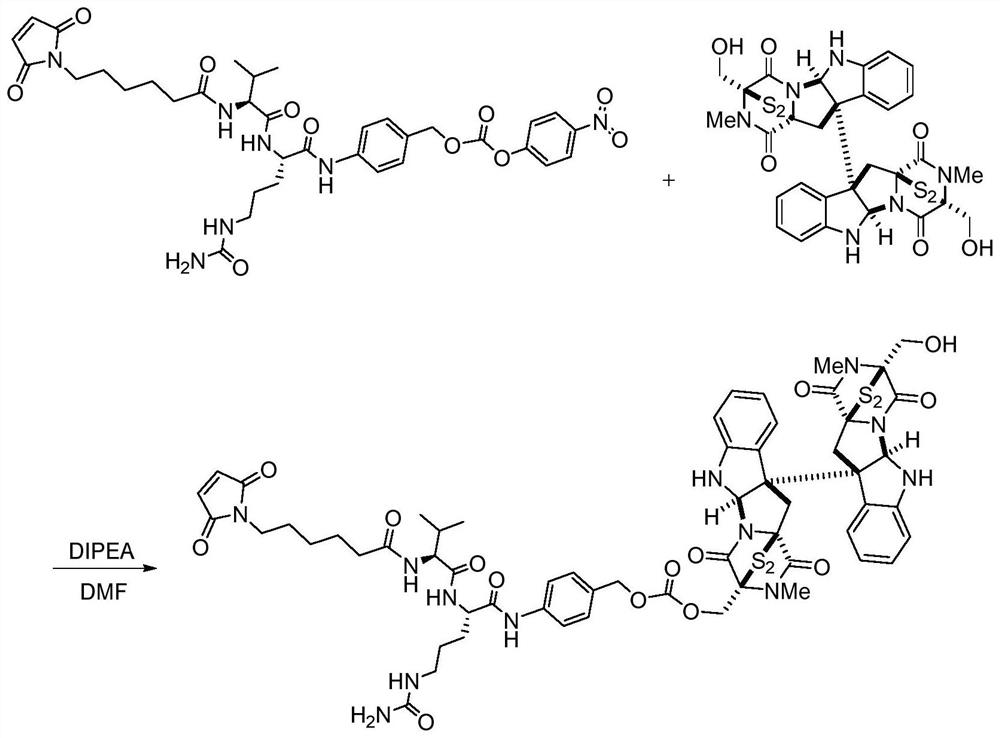
[0049] Embodiment 1: Preparation of vc-Chaetocin drug molecule with linker
[0050] The preparation steps are as follows:
[0051] (1) Mix chaetocin (+)-Chaetocin 15mg (0.022mmol) with N,N-diisopropylethylamine 16.5μL (0.100mmol), 2.4mL N,N-dimethylformamide at room temperature under nitrogen After 20 min of magnetic stirring under protection, take 37 mg (0.050 mmol) of maleimide-modified valine-citrulline dipeptide and 17 μL (0.103 mmol) of N,N-diisopropylethylamine into the reaction solution , stirred overnight at room temperature;
[0052] (2) TLC monitors the completion of the reaction, quenches the reaction solution with 0.40mL 1.3M trifluoroacetic acid and 0.60mL acetic acid, concentrates by rotary evaporation with an oil pump, and purifies by silica gel column chromatography. The eluent is dichloromethane:methanol=6:1 , to obtain 6.8 mg of brown-yellow solid product 6-L-C, yield: 24.4%. 1 H NMR (500MHz, CD 3 OD)δ H (ppm): 8.08 (d, J=6.0Hz, 2H, -CH=CH-), 7.57-7.30 (...
Embodiment 2
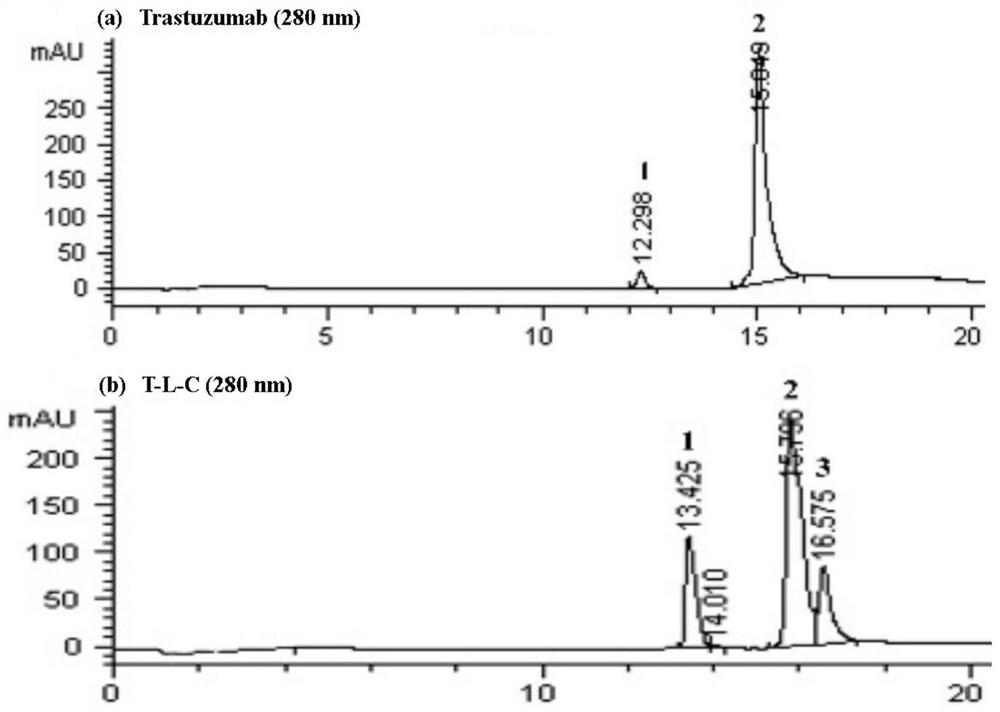
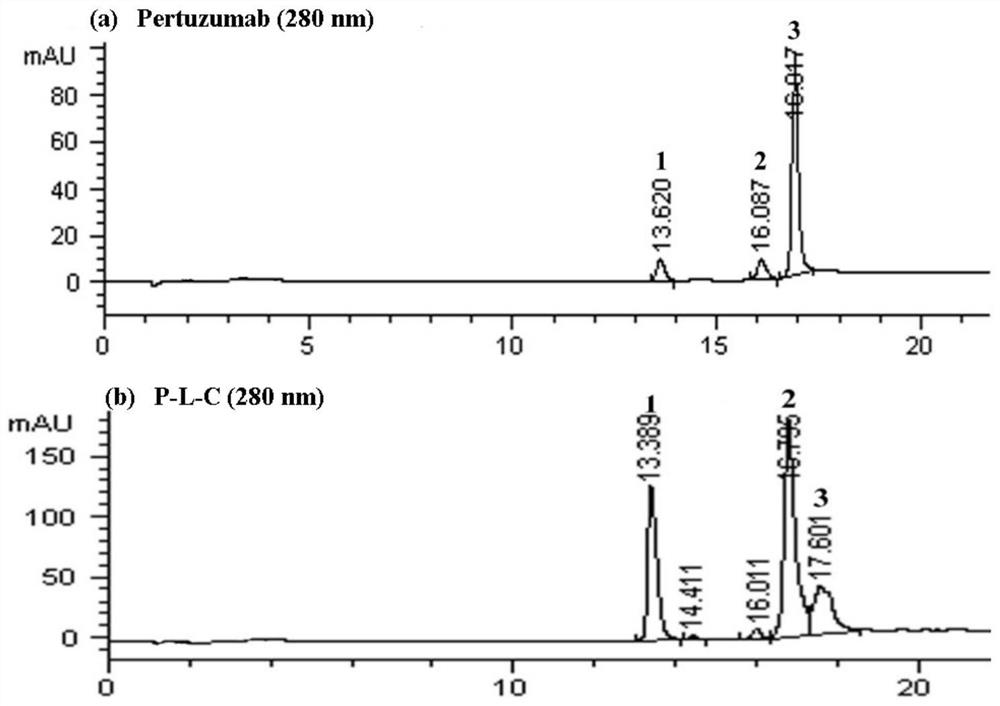
[0054] Example 2: Preparation of Anti-HER2 Antibody-Chaitin Conjugate (T-L-C or P-L-C)
[0055] The preparation method of T-L-C conjugate comprises:
[0056] (1) Replace the trastuzumab antibody solution (20 mg / mL) with PBS reaction buffer (pH 7.4) through a Sephadex G-25 desalting column, and use an ultrafiltration tube to concentrate the monoclonal antibody to adjust the concentration to 10 mg / mL;
[0057] (2) Take 500 μL (10 mg / mL) trastuzumab solution, add 21 μL of 20 mM TCEP solution with a 12-fold excess molar ratio, and incubate at 37 ° C for 1.5 h;
[0058] (3) Use PBS containing 1mM DTPA to pass through a desalting column to purify the reduced monoclonal antibody, add 64 μL of 5.38mM 6-L-C solution with a 10-fold excess molar ratio to the monoclonal antibody solution under ice bath conditions, and let it stand for 1.5h ;
[0059] (4) Add 35 μL of 20 mM cysteine solution with a 20-fold excess molar ratio, let stand for 45 minutes, pass PBS through a desalting colum...
Embodiment 3
[0071] Embodiment 3: in vitro cell proliferation assay biological activity experiment
[0072] In this example, the effects of chaetocin (+)-Chaetocin on the proliferation of various tumor cell lines were studied; with HER2 positive cells SKBR-3, AU565, HER2 negative cells MBA-MD-231 and normal cells HaCaT as objects, The biological activities of Trastuzumab, Pertuzumab monoclonal antibody and T-L-C, P-L-C conjugates were determined.
[0073] In this example, the CCK-8 reagent was used to evaluate the anti-proliferation effect of the drug. The main component of the reagent is water-soluble tetrazolium salt WST-8. WST-8 is bioreduced by intracellular dehydrogenase to generate orange-yellow water-soluble formazan (formazan). The amount of formazan produced is linearly related to the number of living cells. .
[0074] The cell lines selected in this example are: gastric cancer cell SGC-7901, lung cancer cell NCI-H460, liver cancer cell SMMC-7721, breast cancer cell SKBR-3, AU56...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com