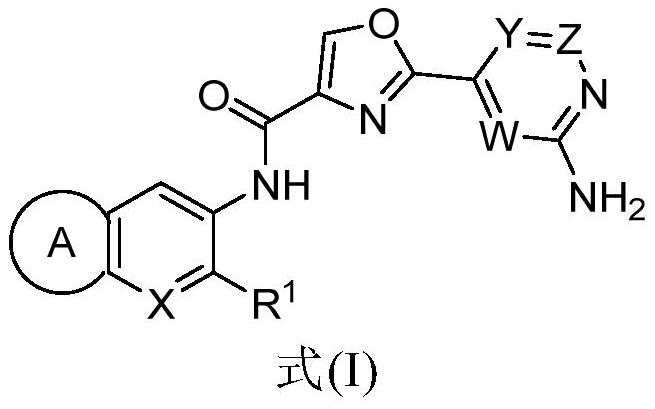
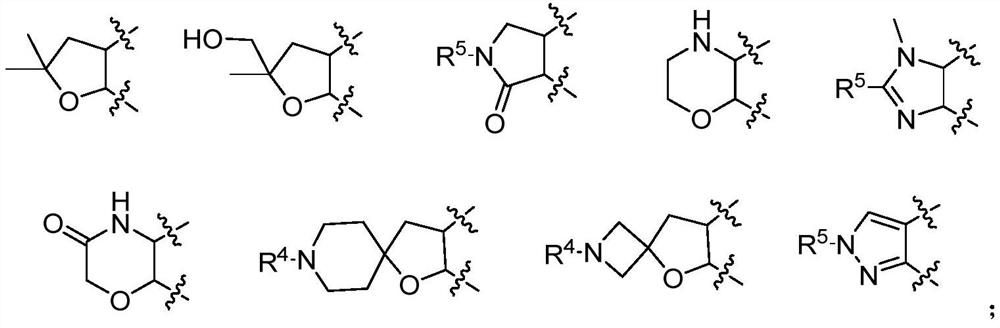
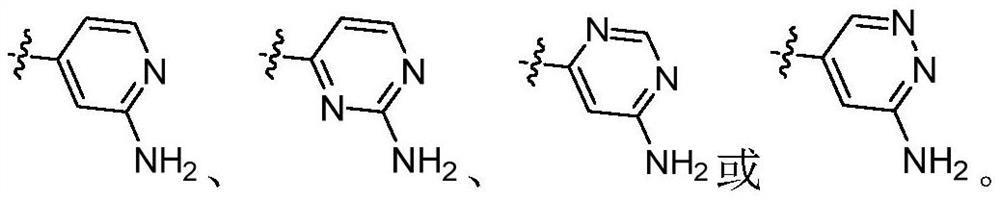
IRAK4 kinase inhibitor as well as preparation and application thereof
A solvate, alkyl technology, applied in the field of medicinal chemistry, can solve the problem of redundant host defense against natural infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] Preparation of compounds of the present invention
[0078] The following reaction schemes illustrate the preparation of the compounds of the present invention.
[0079] Reaction Scheme I
[0080]
[0081] The general synthetic method of a class of compounds of the present invention is shown in Reaction Scheme I. Including the following steps:
[0082] 1. Compound 1a / 1b was obtained by Grignard reaction with methylmagnesium bromide at -78°C to obtain compound 2a / 2b;
[0083] 2. Compound 2a / 2b undergoes a ring-closing reaction under the condition of potassium tert-butoxide to obtain compound 3a / 3b;
[0084] 3. Compound 3a / 3b undergoes nitration reaction under the action of nitric acid to obtain compound 4a / 4b
[0085] 4. Compound 4a undergoes a Suzuki coupling reaction under the action of a palladium catalyst, and compound 4b undergoes a substitution reaction under the action of a base to obtain different forms of formula 5;
[0086] 5. Compound 5 can be reduced u...
Embodiment 1
[0160] 2-(2-aminopyridin-4-yl)-N-(6-(4-(hydroxymethyl)piperidin-1-yl)-2,2-dimethyl-2,3-dihydrobenzo Furan-5-yl)oxazole-4-carboxamide (I-1)
[0161]
[0162] Step 1: Preparation of (1-(2,2-dimethyl-5-nitro-2,3-dihydrobenzofuran-6-yl)piperidin-4-yl)methanol
[0163]
[0164] 6-Fluoro-2,2-dimethyl-5-nitro-2,3-dihydrobenzofuran (200 mg, 94.7 μmol), 4-hydroxymethylpiperidine (218 mg, 1.89 mmol) were dissolved in N , N-dimethylformamide (5mL), potassium carbonate (262mg, 1.89mmol) was added, reacted at 40°C for two hours, TLC detected that the reaction was complete, added ethyl acetate (25mL) and water for extraction, the ethyl acetate layer was washed with water, Wash with saturated brine, dry over anhydrous sodium sulfate, filter with suction, concentrate to dryness, and put directly into the next reaction.
[0165] Step 2: Preparation of (1-(5-amino-2,2-dimethyl-2,3-dihydrobenzofuran-6-yl)piperidin-4-yl)methanol
[0166]
[0167] Dissolve the reaction product of the p...
Embodiment 2
[0175] (R)-N-(6-(3-aminopiperidin-1-yl)-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)-2-(2-amino Pyridin-4-yl)oxazole-4-carboxamide trifluoroacetate (I-2)
[0176]
[0177] Step 1: R)-(4-(4-((6-(3-((tert-butoxycarbonyl)amino)piperidin-1-yl)-2,2-dimethyl-2,3-di Preparation of tert-butyl hydrobenzofuran-5-yl)carbamoyl)oxazol-2-yl)pyridin-2-yl)carbamate
[0178]
[0179] (R)-(4-(4-((6-(3-((tert-butoxycarbonyl)amino)piperidin-1-yl)-2,2 -Dimethyl-2,3-dihydrobenzofuran-5-yltert-butyl)carbamoyl)oxazol-2-yl)pyridin-2-yl)carbamate tert-butyl. 1 H NMR (400MHz, Chloroform-d) δ10.15(s,1H),8.65(s,1H),8.41(d,J=5.2Hz,1H),8.37(s,1H),8.31(s,1H) ,7.99(s,1H),7.63(dd,J=5.2,1.5Hz,1H),6.60(s,1H),3.14(s,1H),3.02(s,2H),2.77(s,3H), 1.90(s,1H),1.67(s,3H),1.55(s,9H),1.47(s,6H),1.19(s,9H).
[0180] Step 2: (R)-N-(6-(3-aminopiperidin-1-yl)-2,2-dimethyl-2,3-dihydrobenzofuran-5-yl)-2-( Preparation of 2-aminopyridin-4-yl)oxazole-4-carboxamide trifluoroacetate
[0181] (R)-(4-(4-((6-(3-((tert-butoxyca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com