O-carborane compound composition for assisting BNCT
A compound composition and o-carborane technology, which is applied in the directions of drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., to achieve the effect of obvious enrichment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
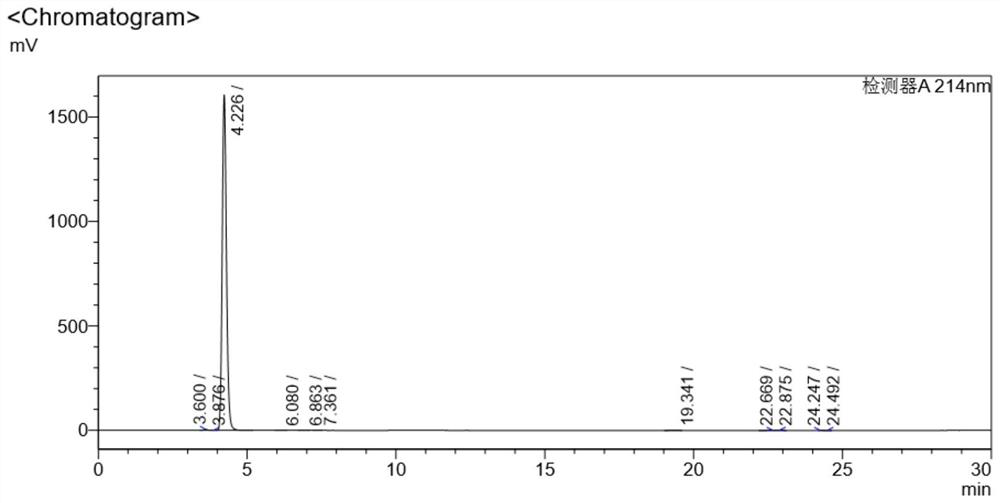
[0027] Add 10 grams of vitamin B12 crude product (content 96.512%) and 100 grams of methyl alcohol into a 500 milliliter round bottom flask, after cooling to 0-5 degrees, add 1 gram of sodium cyanoborohydride in 3 times, stir at room temperature for 1 hour, Filtration and vacuum drying at 50°C for 12 hours yielded 8.6 g of pure vitamin B12 with a yield of 86% and a purity greater than 99.674%. Chromatogram see figure 2 , see the data in the table below
[0028] Detector A214nm
[0029] Peak# Ret. Time area Height area% Conc. unit 1 3.600 44751 5051 0.180 0.180 2 3.876 3081 414 0.019 0.019 3 4.226 15886874 1606650 99.674 99.674 4 6.080 4291 385 0.027 0.027 5 6.863 3605 287 0.023 0.023 6 7.361 1221 95 0.008 0.008 7 19.341 10634 434 0.067 0.067 8 22.669 10720 467 0.067 0.067 9 22.875 3862 352 0.024 0.024 10 24.247 2392 182 0.015 0.015 11 24.49...
Embodiment 2
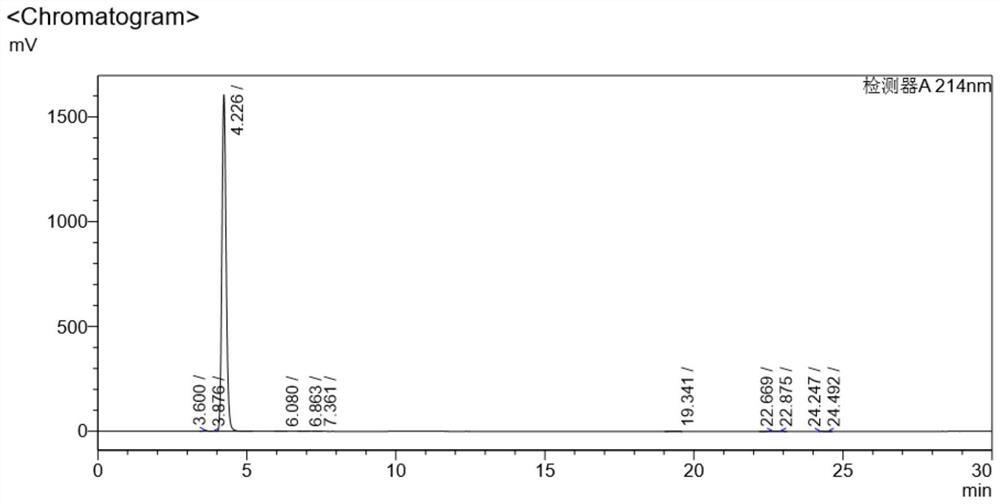
[0031] Add 10 grams of vitamin B12 crude product (content 96.512%) and 100 grams of ethanol into a 500 milliliter round bottom flask, after cooling down to 0-5 degrees, add 1 gram of sodium cyanoborohydride in 3 times, stir at room temperature for 2 hours, Filtration and vacuum drying at 50°C for 12 hours yielded 8.9 g of pure vitamin B12 with a yield of 89% and a purity greater than 99.464%. Chromatogram see image 3 , see the data in the table below
[0032] Detector A214nm
[0033] Peak# Ret. Time area Height area% Conc. unit 1 3.600 44751 5051 0.280 0.280 2 3.876 3081 414 0.019 0.019 3 4.226 15886874 1606650 99.464 99.464 4 6.080 4291 385 0.027 0.027 5 6.863 3605 287 0.023 0.023 6 7.361 1221 95 0.008 0.008 7 19.341 10634 434 0.067 0.067 8 22.669 10720 467 0.067 0.067 9 22.875 3862 352 0.024 0.024 10 24.247 2392 182 0.015 0.015 11 24.492 ...
Embodiment 3
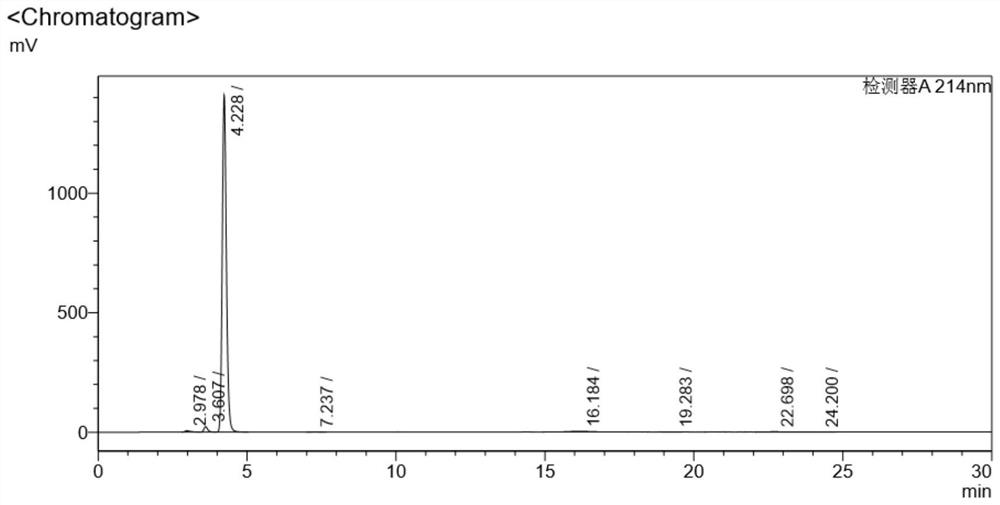
[0035] (Blank experiment without adding sodium cyanoborohydride) 10 grams of vitamin B12 crude product (content 96.512%) and 100 grams of ethanol were added in a 500 ml round-bottomed flask, cooled to 0-5 degrees, and then stirred at room temperature for 2 hours. Filtration and vacuum drying at 50°C for 12 hours yielded 9.9 g of pure vitamin B12, with a yield of 99% and a purity of 96.912%. Chromatogram see Figure 4 , see the data in the table below
[0036] Detector A214nm
[0037] Peak# Ret. Time area Height area% Conc. unit 1 2.978 71287 5899 0.313 0.313 2 3.607 202610 22760 1.431 1.431 3 4.228 13668896 1410020 96.912 96.912 4 7.237 5171 332 0.037 0.037 5 16.184 148841 2561 1.051 1.051 6 19.283 7422 285 0.052 0.052 7 22.698 43870 1228 0.310 0.310 8 24.200 14838 452 0.105 0.105 total 14162934 1443537 100.000
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com