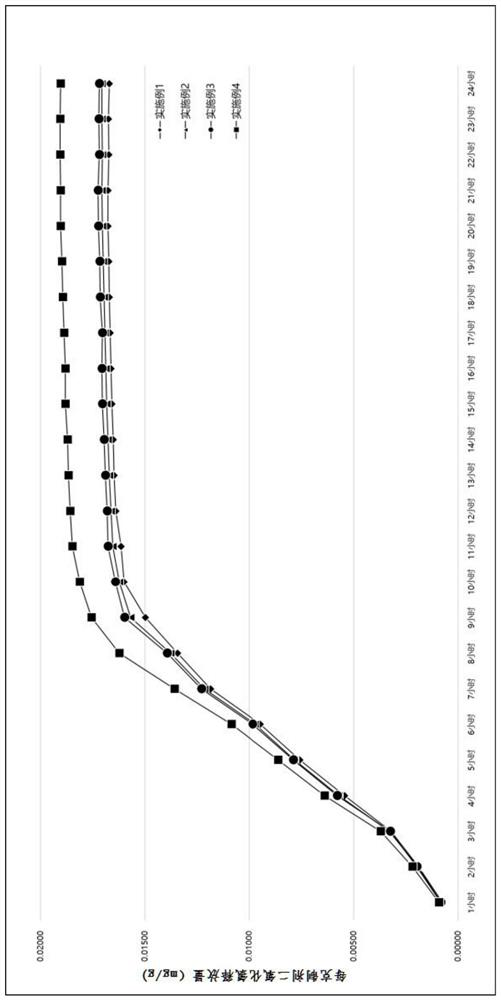
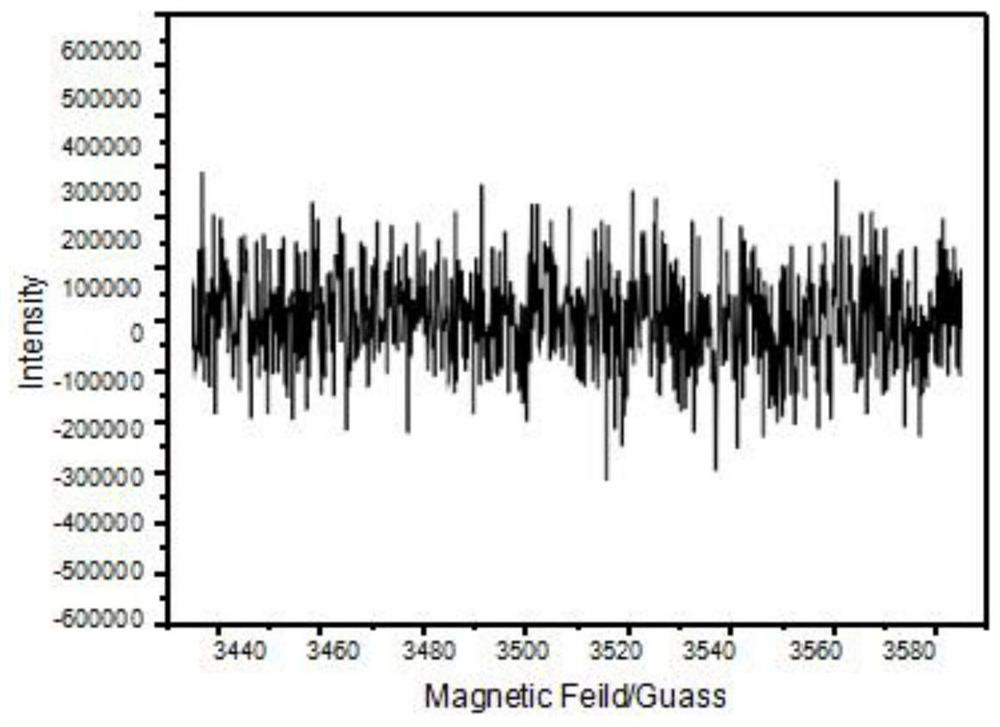
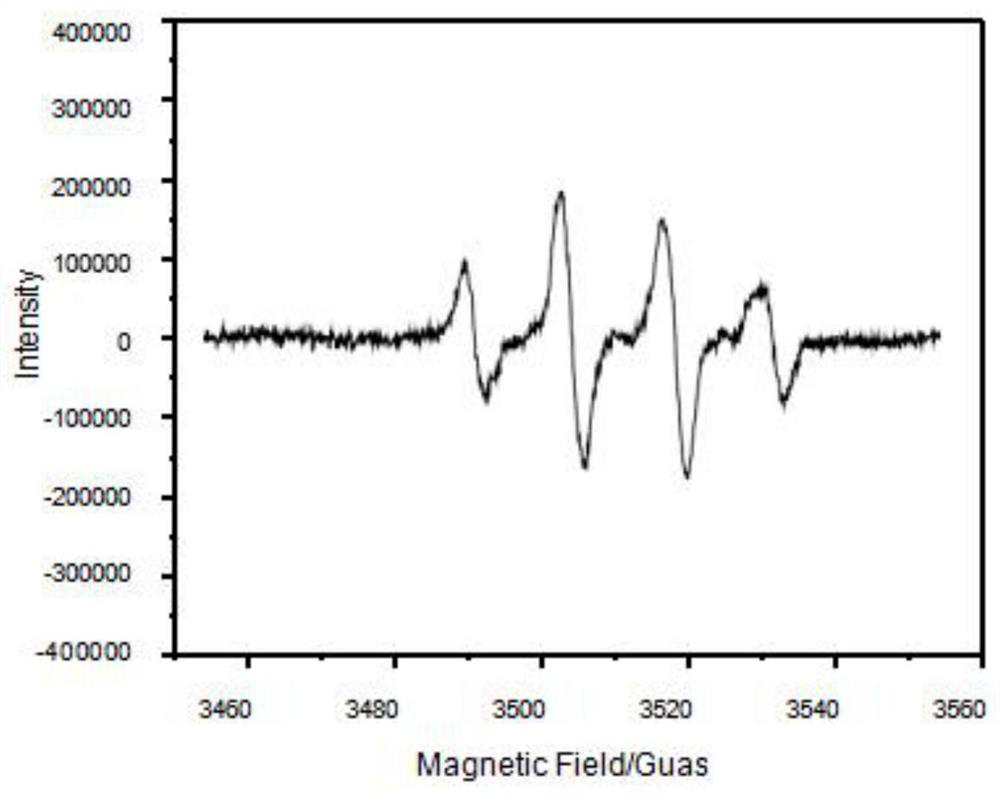
Adsorbent and preparation method thereof, chlorine dioxide preparation and preparation method and application of chlorine dioxide preparation
A chlorine dioxide and adsorbent technology, applied in the field of chlorine dioxide preparation, can solve the problems of unsafe, particularly prominent impact, not widely used and mature in the market, and achieve huge social and economic benefits.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The invention provides a kind of preparation method of adsorbent, comprises the following steps:
[0027] mixing the ammonium fluoroborate solution with diatomite, and performing a modification reaction to obtain modified diatomite;
[0028] Mixing the modified diatomite, kaolin and bentonite to obtain a composite carrier;
[0029] The composite carrier is calcined to obtain the adsorbent.
[0030] In the invention, the ammonium fluoroborate solution is mixed with the diatomite, and the modification reaction is carried out to obtain the modified diatomite. In the present invention, the mass concentration of the ammonium fluoroborate solution is preferably 5-8%, more preferably 8%; the pH value of the ammonium fluoroborate solution is preferably 9.5-10.3. In the present invention, sodium hydroxide and / or dilute sulfuric acid are preferably used to adjust the pH value of the ammonium fluoroborate solution. In the present invention, the particle size of the diatomite is...
Embodiment 1
[0044] 1. Preparation of adsorbent:
[0045] 1) Weigh a certain amount of diatomite (100-200 mesh) and place it in a Erlenmeyer flask; 2) Weigh a certain amount of ammonium fluoroborate into a beaker, stir with a glass rod until completely dissolved to obtain a 5wt.% ammonium fluoroborate solution , adjust the pH value of the solution to 9.5-10; 3) According to the ratio of 1:2 (100g diatomaceous earth: 200mL ammonium fluoroborate solution), add the ammonium fluoroborate solution dropwise to the three-necked flask under stirring conditions, at 90 ℃ 4) Weigh a certain amount of kaolin and bentonite (both particle diameters are 100-200 mesh), and mix them according to the mass ratio of 5:1:1 (modified diatomite (soil: kaolin: bentonite) into the reaction kettle, and continue to stir for 3 hours; 5) then dry in a vacuum oven for 2 hours; 6) after drying, roast in a muffle furnace at 500°C for 5 hours to obtain the adsorbent.
[0046] 2. Preparation of chlorine dioxide preparatio...
Embodiment 2
[0050] 1. Preparation of adsorbent:
[0051] 1) Weigh a certain amount of diatomite (100-200 mesh) and place it in a Erlenmeyer flask; 2) Weigh a certain amount of ammonium fluoroborate into a beaker, stir with a glass rod until completely dissolved to obtain 8wt.% ammonium fluoroborate solution, Adjust the pH value of the solution to 9.5-10; 3) According to the ratio of 1:2 (100g diatomaceous earth: 200mL ammonium fluoroborate solution), add the ammonium fluoroborate solution dropwise to the three-necked flask under stirring conditions. 4) Weigh a certain amount of kaolin and bentonite (both with a particle size of 100-200 mesh), and mix them according to the ratio of 5:1:1 (modified diatomite: kaolin) : bentonite) into the reaction kettle, continue to stir for 3h; 5) then dry in a vacuum oven for 2h; 6) after drying, roast in a muffle furnace at 500°C for 5h to obtain the adsorbent.
[0052] 2. Preparation of chlorine dioxide preparation:
[0053] 1) Preparation of stable ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com