RNA vaccine for treating non-small cell lung cancer and construction method thereof
A non-small cell lung cancer and vaccine technology, applied in DNA/RNA vaccination, complete cell/virus/DNA/RNA components, vaccines, etc., to achieve good therapeutic effect, good stability, and easy delivery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Neoantigen Screening
[0038] 1. Construction and sequencing of animal models
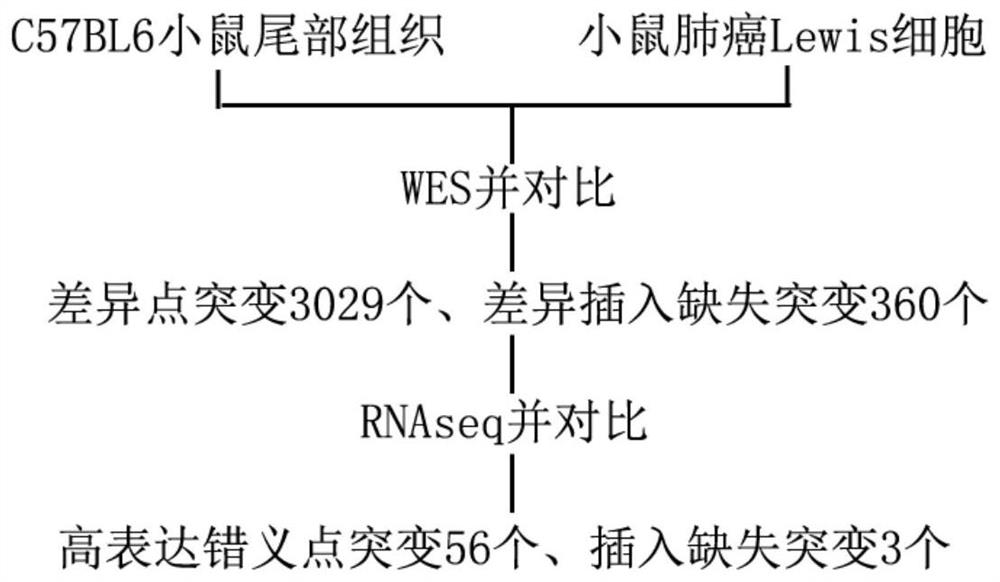
[0039] Detection of tumor-specific non-synonymous mutations. The first step in identifying tumor neoantigens is to find non-synonymous mutations unique to tumor cells. With the advances in sequencing technology, it is not difficult to perform whole-genome sequencing (WGS) or whole-exome sequencing (WES) simultaneously on tumor tissue and normal tissue of the same individual. By comparing the results of the two, non-synonymous mutation sites unique to tumor tissues can be obtained, including point mutations, insertion-deletion mutations, and frameshift mutations. The latest research shows that, combined with RNA sequencing (RNAseq) technology, it can further prompt the expression of non-synonymous mutations from the transcription level and provide assistance for the screening of neoantigens.
[0040] The present invention conducts research on advanced NSCLC, selects C57BL6 mice a...
Embodiment 2
[0056] The construction of embodiment 2 neoantigen RNA vaccine (for the treatment of the RNA vaccine of non-small cell lung cancer)
[0057] In order to construct an RNA vaccine, in vitro transcription (IVT) of its full nucleotide sequence (DNA sequence) needs to be achieved.
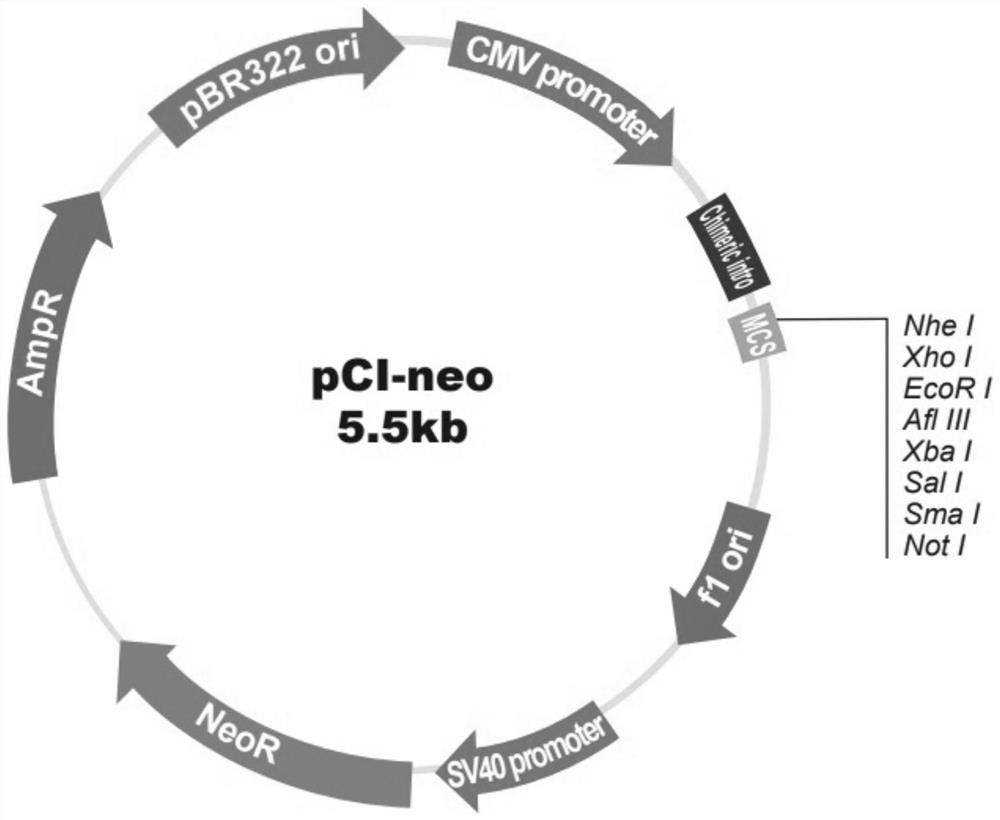
[0058] Select the pCI-neo (pCI-neo mammalian expression vector) plasmid as the vector, first insert the DNA sequence of the designed RNA vaccine into the pCI-neo plasmid between the XhoI and NotI restriction sites, and amplify the plasmid loaded with the RNA vaccine sequence . Schematic diagram of the structure of pCI-neo, see figure 2 .
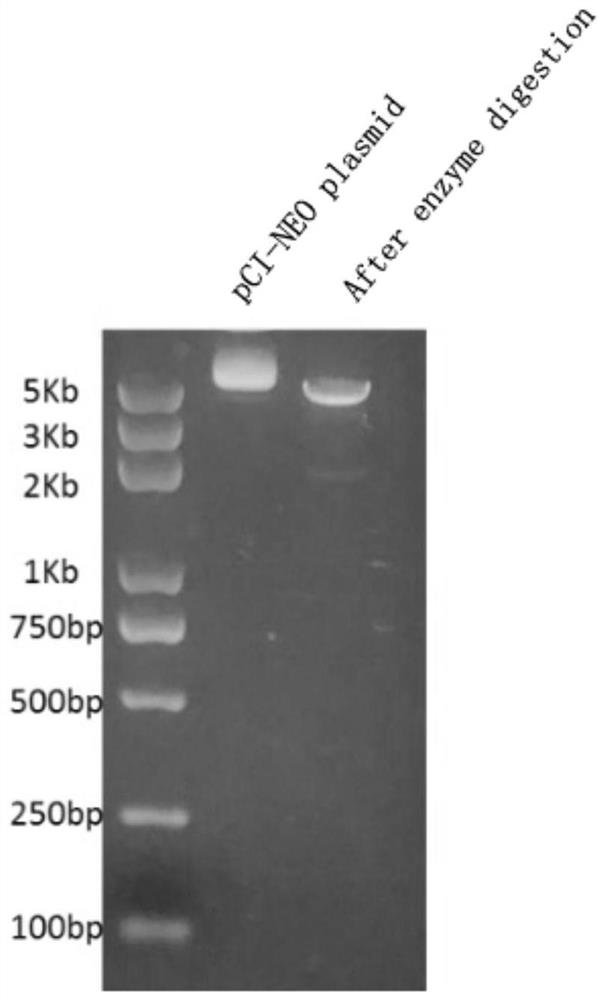
[0059] The plasmid carrying the RNA vaccine sequence was cut with Sap I endonuclease to make it linearized. After linearization, it was also verified by agarose gel electrophoresis. The results are as follows image 3 , indicating that the Sap I endonuclease has successfully linearized the plasmid and the in vitro transcription step can begin. Subsequent use of i...
Embodiment 3
[0062] Example 3 Construction of PEG--PLL--PLLeu self-assembled micelle carrier
[0063] Because of the structural specificity of RNA vaccines, its function is closely related to the delivery method. The RNA vaccine of the present invention is wrapped by nanoparticles and administered intravenously, and the effect is better: on the one hand, the nanoparticles can protect the loaded RNA from being degraded and destroyed; on the other hand, the physical and chemical properties of the nanoparticles can realize slow, Effects of sustained-release RNA vaccines. In addition, secondary lymphoid organs such as lymph nodes and spleen are important places for the identification and activation of T cells and DC cells, and intravenous delivery of nanoparticles is conducive to the distribution of RNA vaccines in secondary lymphoid organs throughout the body.
[0064] (1) Synthesis of PEG-PLL-PLLeu
[0065] Polyethylene glycol-polylysine-polyleucine (PEG-PLL-PLLeu) can self-assemble to for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com