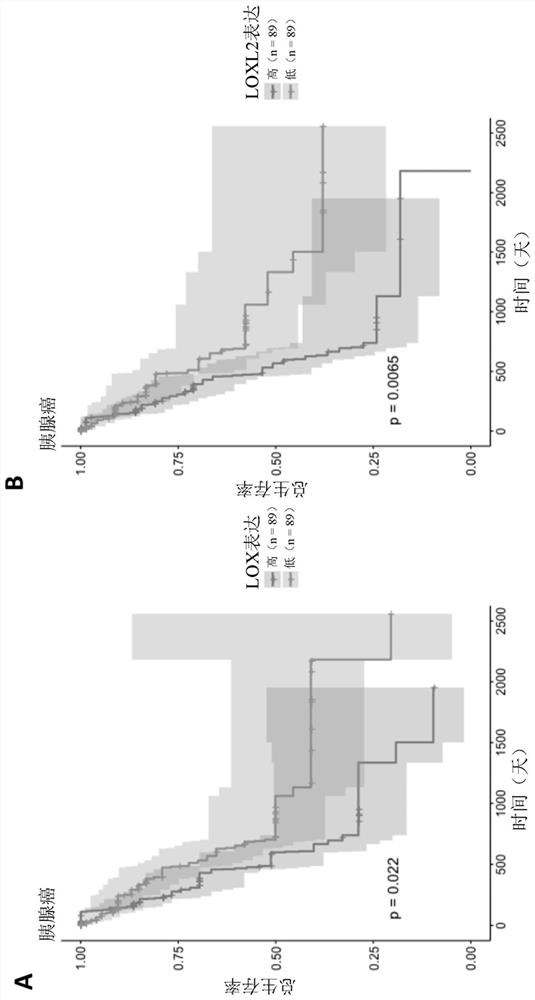
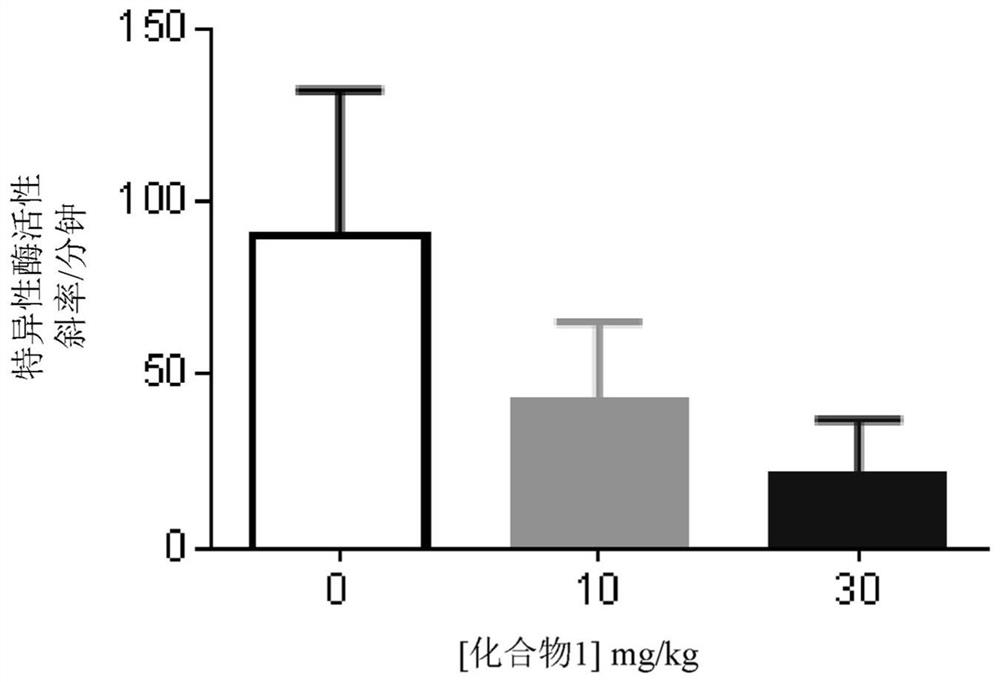
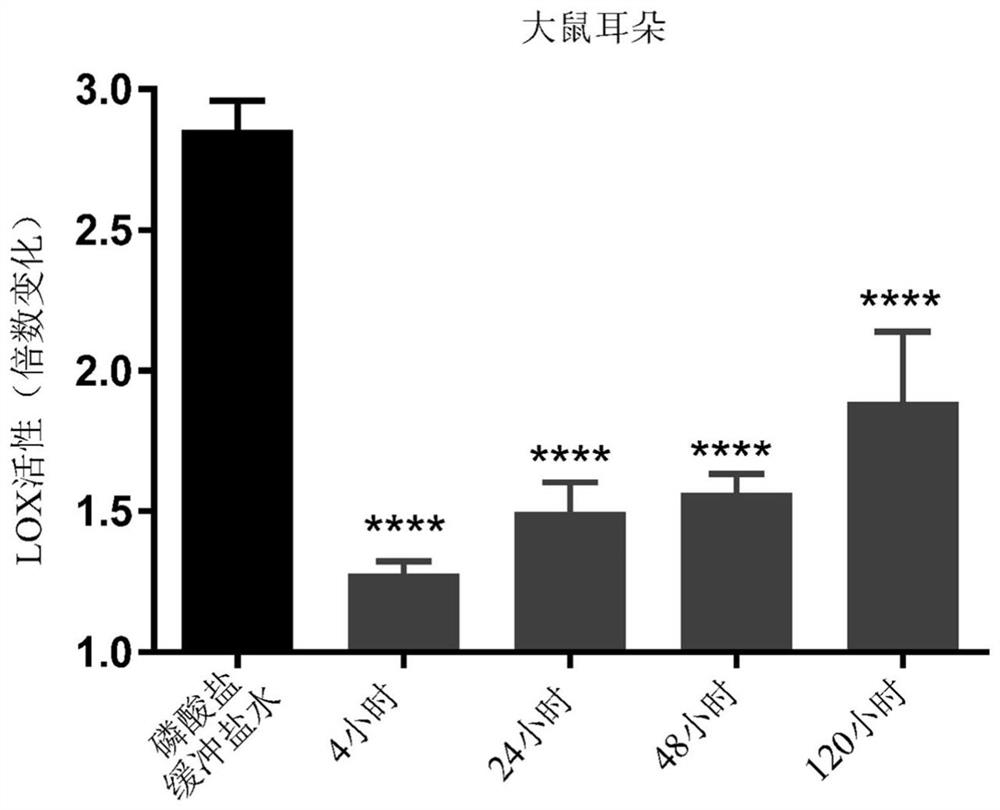
Haloallylamine sulfone derivative inhibitors of lysyl oxidases and uses thereof
A halogen and compound technology, applied in the direction of amide active ingredients, medical preparations containing active ingredients, organic chemistry, etc., can solve problems such as poor tolerance of BAPN
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0278] The preparation of formula I compound
[0279] Compounds of formula I can be prepared by those skilled in the art using methods and materials known in the art and with reference to standard textbooks such as "Advanced Organic Chemistry" by Jerry March (Third Edition, 1985, John Wiley and Sons) or Richard C. . Larock's "Comprehensive Organic Transformations (Comprehensive Organic Transformations)" (1989, VCH Press) to easily prepare.
example 1
[0431] Preparation of tert-butyl (Z)-(4-bromo-3-fluorobut-2-en-1-yl)carbamate
[0432]
[0433] Procedure A: Preparation of tert-butyl 2-oxoethylcarbamate
[0434]
[0435] To a stirred solution of 3-amino-1,2-propanediol (20.0 g, 0.22 mol) in water (200 mL) was added di-tert-butyl dicarbonate (55.5 mL, 0.24 mol) at 0-5°C . After adjusting the alkalinity of the solution to pH about 9 by addition of aqueous solution, the mixture was stirred at room temperature (rt) for 18 hours by addition of NaOH (6N). The reaction mixture was cooled to 0-5°C, then acidified to pH about 6, then sodium metaperiodate (56.3 g, 0.26 mol) was added. The resulting suspension was stirred at room temperature for 2 hours. The mixture was filtered to remove all solids, and the filtrate was transferred to a separatory funnel and extracted with ethyl acetate (200 mL). Sodium chloride was added to the aqueous layer until a saturated solution was obtained. The aqueous layer was then further ext...
example 2
[0446] The following compounds were prepared according to Procedures E, F, G, H and I.
[0447] Preparation of (Z)-4-((2-((4-amino-2-fluorobut-2-en-1-yl)sulfonyl)phenoxy)methyl)-N,N-diisopropylbenzene Sulfonamide hydrochloride (compound 11)
[0448]
[0449] Procedure E: Preparation of 4-(bromomethyl)-N,N-diisopropylbenzenesulfonamide
[0450]
[0451] To stirred 4-(bromomethyl)benzenesulfonyl chloride (500 mg, 1.86 mmol) in CH at 0 °C 2 Cl 2 (10 mL) was added dropwise diisopropylamine (0.65 mL, 4.63 mmol). After the addition, the resulting mixture was stirred at this temperature for 30 minutes, then allowed to warm to room temperature and stirred for a further 48 hours. The reaction mixture was dissolved in HCl (1M, 20mL) and CH 2 Cl 2 (20mL). The organic layer was washed with aqueous HCl (1M; 20 mL) and water (20 mL), washed with Na 2 SO 4 Drying and concentration in vacuo afforded the title compound (yellow oil, 190 mg) in a mixture with 4-(chloromethyl)-N,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com