Method for treating acidic mine wastewater containing heavy metal ions
A technology for acid mine wastewater and heavy metal ions, which is applied in the fields of filtration treatment, water/sewage treatment, mining wastewater treatment, etc. It can solve the problems of high energy consumption, complex process and high cost of magnetization and heating
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
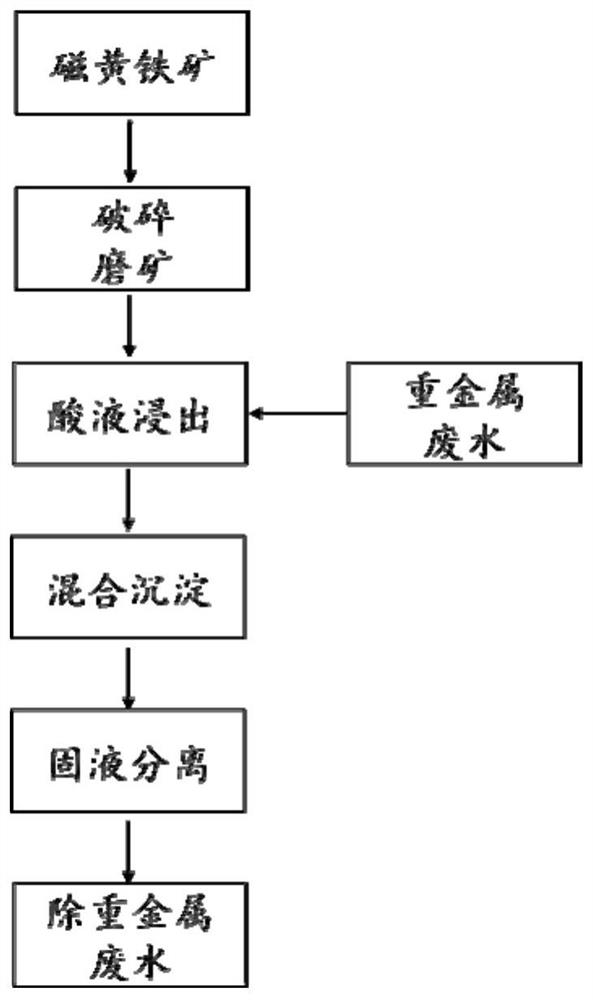
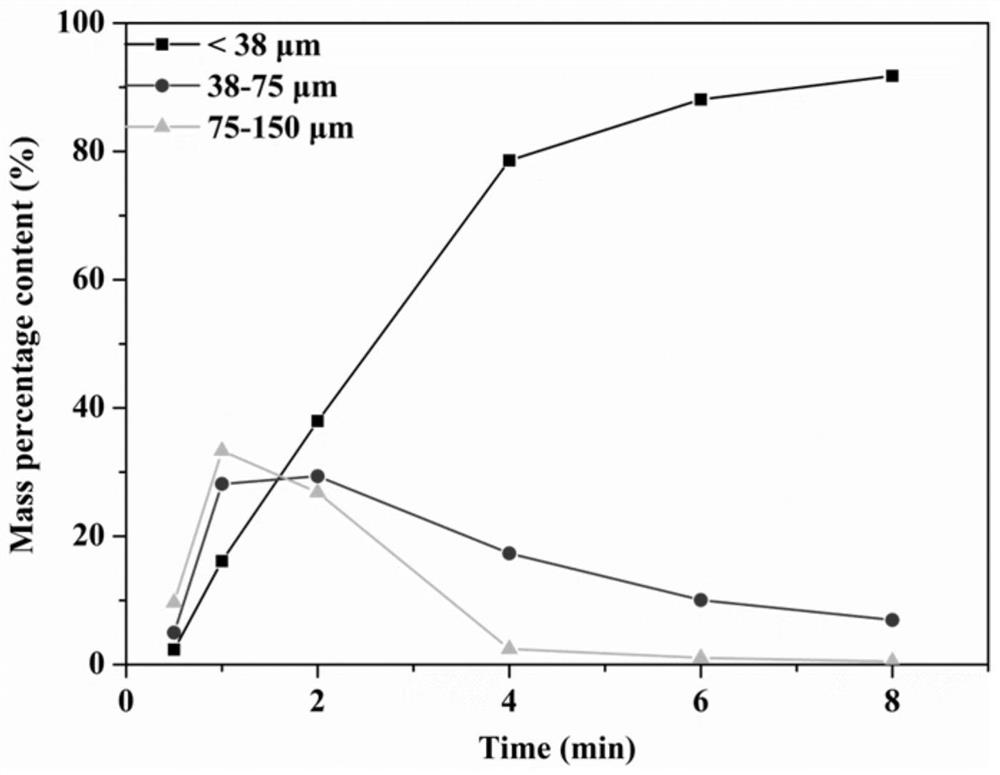
[0060] (1) Artificially crushing the natural pyrrhotite, then using a three-head grinder to grind for 2 minutes to obtain pyrrhotite particles with a particle size of less than 150 μm;
[0061] (2) Take 1g pyrrhotite particles and 5mol L -1 h 2 SO 4 Perform mixing and stirring reaction at 25°C, and control the liquid-solid ratio of pyrrhotite and acid to 20mL g -1 , The acid solution dripping time is 30min.
[0062] (3) passing the generated hydrogen sulfide into pH 3.5, Pb 2+ The concentration is 25mg L -1 The mixed precipitation reaction was carried out in the simulated acid wastewater, the reaction temperature was 25°C, and the reaction time was 6h.
[0063] (4) The acid waste water and the lead sulfide precipitation are separated into solid and liquid by a filtration method to obtain the lead sulfide precipitation particles and the lead removal waste water.
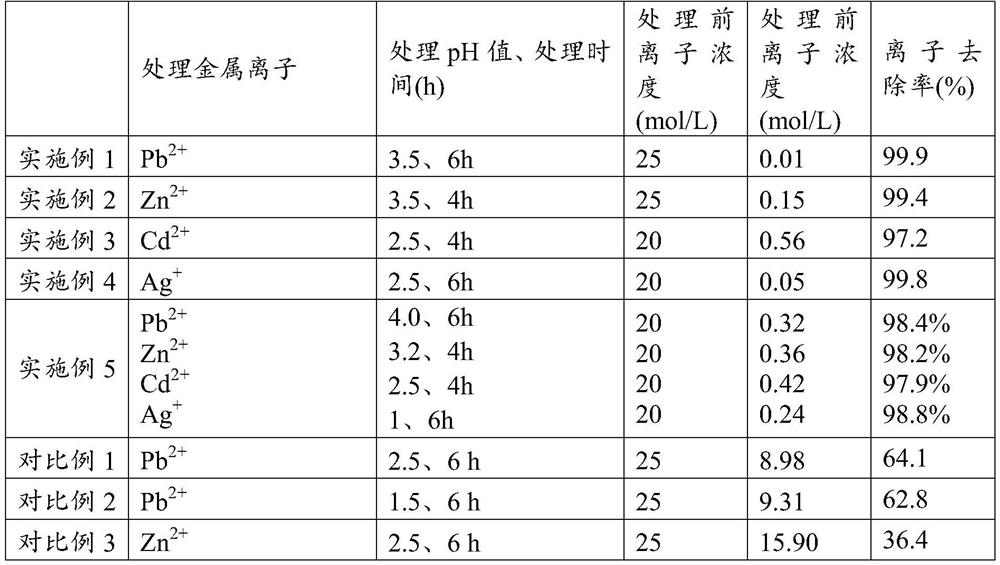
[0064] After testing, Pb in the treated wastewater 2+ The concentration is 0.01mg L -1 , The removal rate r...
Embodiment 2
[0066] (1) Artificially crushing the natural pyrrhotite, then using a three-head grinder to grind for 2 minutes to obtain pyrrhotite particles with a particle size of less than 150 μm;
[0067] (2) Take 1g pyrrhotite particles and 5mol L -1 h 2 SO 4 Perform mixing and stirring reaction at 25°C, and control the liquid-solid ratio of pyrrhotite and acid to 20mL g -1 , The acid solution dripping time is 30min.
[0068] (3) Pass the generated hydrogen sulfide into pH 3.5, Zn 2+ The concentration is 25mg L -1 The mixed precipitation reaction was carried out in the wastewater, the reaction temperature was 25°C, and the reaction time was 4h.
[0069] (4) Separating the acidic wastewater and the zinc sulfide precipitation into solid and liquid to obtain zinc sulfide precipitation particles and zinc removal wastewater.
[0070] After testing, Zn in the treated wastewater 2+ The concentration is 0.15mg L -1 , The removal rate reaches 99.4%.
Embodiment 3
[0072] (1) Artificially crushing the natural pyrrhotite, using a three-head grinder to grind the ore, and using a vibrating screen to sieve to obtain pyrrhotite particles with a particle size of 38-150 μm;
[0073] (2) Take 1g pyrrhotite particles and 5mol L -1 h 2 SO 4 Perform mixing and stirring reaction at 25°C, and control the liquid-solid ratio of pyrrhotite and acid to 20mL g -1 , The acid solution dripping time is 30min.
[0074] (3) Pass the produced hydrogen sulfide into pH 2.5, Cd 2+ The concentration is 20mg L -1 The mixed precipitation reaction was carried out in the simulated acid wastewater, the reaction temperature was 25°C, and the reaction time was 4h.
[0075] (4) Separating the acidic wastewater and the cadmium sulfide precipitation into solid and liquid to obtain cadmium sulfide precipitation particles and cadmium removal wastewater.
[0076] After testing, the Cd in the treated wastewater 2+ The concentration is 0.56mg L -1 , The removal rate reach...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com