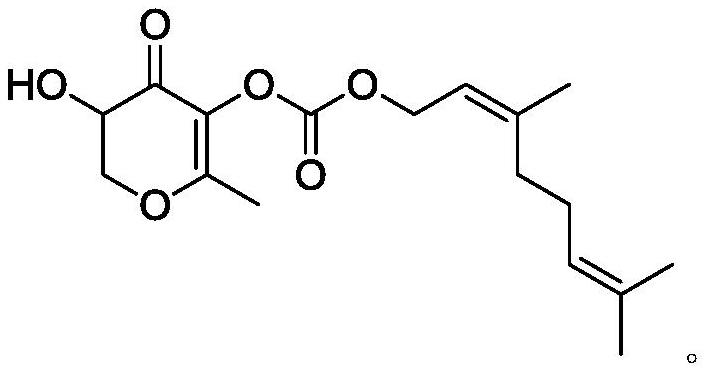
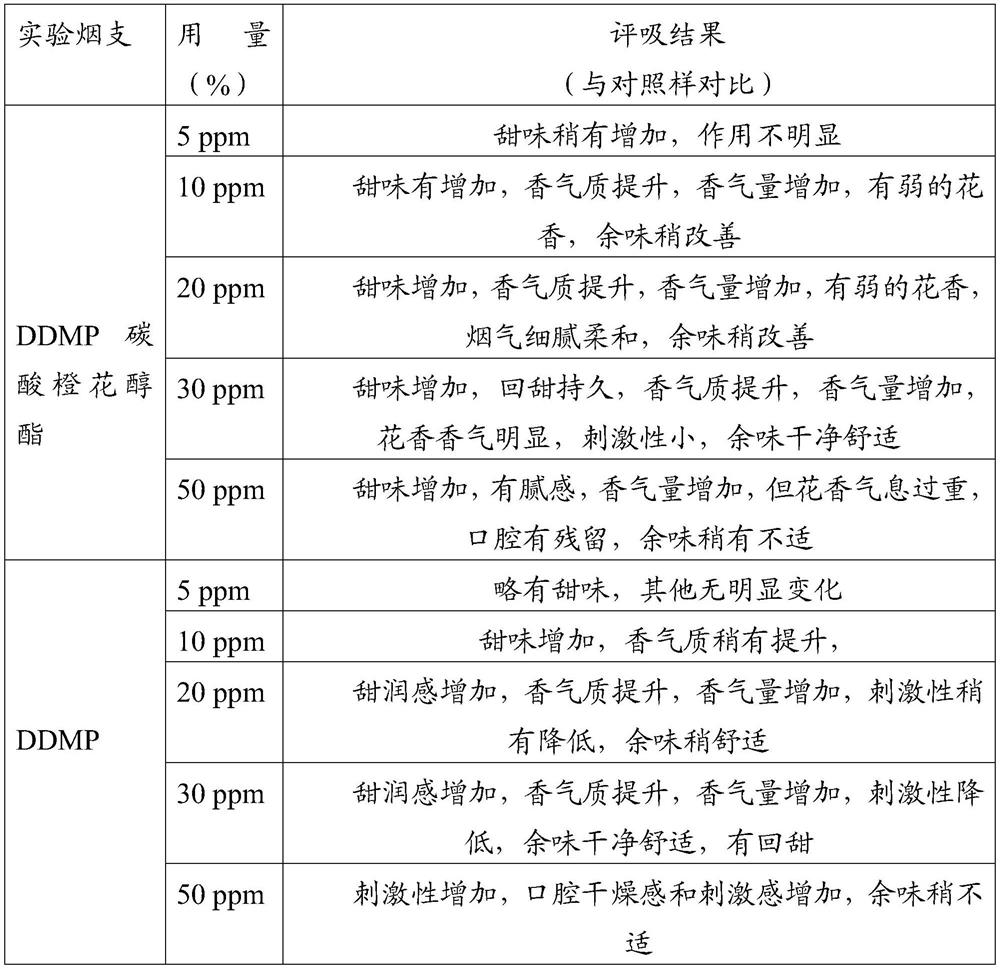
2, 3-dihydro-3-hydroxy-6-methyl-4H-pyran-4-one-5-O-carbonic acid nerol ester and application thereof
A technology of nerol ester and nerol, applied in the field of cigarette flavor synthesis, can solve the problems of easy deterioration and damage, poor stability of DDMP and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Under nitrogen protection, nerol (1.54g, 10mmol) was dissolved in 30mL of anhydrous dichloromethane, the reaction system was cooled to 0°C, and triethylamine (2.42g, 24mmol) and trichloromethyl chloroformate ( 0.98g, 3.3mmol), after the addition, react at room temperature for 2h, then add 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (1.44g , 10mmol), continue the reaction at room temperature for 3h, wash the reaction solution with saturated sodium chloride solution after the reaction, separate the organic layer, dry over anhydrous sodium sulfate, evaporate the solvent, and the residue is purified by silica gel column chromatography, petroleum ether ( V): Elution with ethyl acetate (V)=5:1 to obtain 2.66g of the target product 2,3-dihydro-3-hydroxyl-6-methyl-4H-pyran-4-one-5-O- Neryl carbonate (DDMP neryl carbonate), yield 82.6%.
Embodiment 2
[0023] Under nitrogen protection, nerol (1.54g, 10mmol) was dissolved in 30mL of anhydrous chloroform, the reaction system was cooled to 0°C, and pyridine (2.37g, 30mmol) and trichloromethyl chloroformate (0.98g ,3.3mmol), after the addition, react at room temperature for 2h, then add 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one (1.44g, 10mmol ), continue to react for 3h at room temperature, wash the reaction solution with saturated sodium chloride solution after the reaction, separate the organic layer, dry over anhydrous sodium sulfate, evaporate the solvent, and the residue is purified by silica gel column chromatography, petroleum ether (V) : Elution with ethyl acetate (V) = 5:1 to obtain 2.56g of target 2,3-dihydro-3-hydroxy-6-methyl-4H-pyran-4-one-5-O-orange carbonate Ethyl alcohol ester (DDMP neryl carbonate), yield 79.0%.
Embodiment 3
[0025] Under nitrogen protection, dissolve nerol (1.54g, 10mmol) in 30mL of anhydrous 1,2-dichloroethane, cool the reaction system to 0°C, and add diisopropylethylamine (3.88g, 30mmol) in sequence and trichloromethyl chloroformate (1.19g, 4mmol), react at room temperature for 3h after the addition, and then add 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran to the reaction system -4-ketone (1.44g, 10mmol), continue to react at room temperature for 3h, wash the reaction solution with saturated sodium chloride solution after the reaction, separate the organic layer, dry over anhydrous sodium sulfate, evaporate the solvent, and pass the residue through silica gel Purified by column chromatography, eluting with petroleum ether (V): ethyl acetate (V) = 5:1, to obtain 2.24 g of the target product 2,3-dihydro-3-hydroxy-6-methyl-4H-pyran-4 - Keto-5-O-nerol carbonate (DDMP nerol carbonate), yield 69.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com