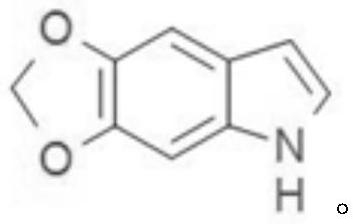
Preparation method of 5, 6-methylenedioxy indole
A technology of methylenedioxyindole and dimethoxy, which is applied in the field of preparation of 5,6-methylenedioxyindole, can solve problems such as unsuitability for large-scale production and complicated operation, and achieve High productivity, simple operation, and cost reduction effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
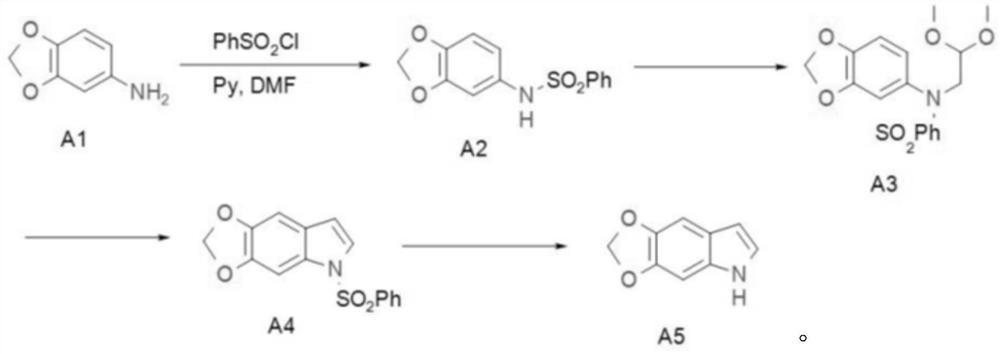
[0026] The technical scheme of the present invention is specifically described below in conjunction with the examples, and the present invention discloses a preparation method of 5,6-methylenedioxyindole
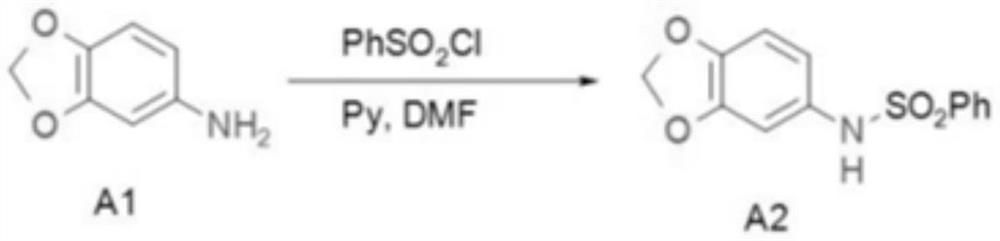
[0027] first step reaction
[0028]
[0029] Add 48Kg DMF, 3.6kg A1 and 3.1kg pyridine to the 100L kettle in sequence, and add 6.55kg benzenesulfonyl chloride dropwise at a temperature of 80-100°C. Insulate at 80°C for 24h. HPLC detects that A1 disappears, washed with 30kg of water, 24kg of 2N hydrochloric acid, and 10kg of water. Spin dry, beat with 8 kg of ether, filter, spin dry the filtrate and beat with 1 kg of petroleum ether, combine the filter cakes twice, and dry to obtain 7.2 kg of rose-red solid. The yield was 95%, and the HPLC purity was 99%.
[0030] second step reaction
[0031]
[0032] Add 13kg THF to the kettle, and slowly add 4.25kg sodium hydrogen. Dissolve 7.2kg of A2 in 20kg of THF and add it dropwise into the kettle, keeping the temperature b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com