Method for evaluating quality of blood samples
A blood sample and molecular technology, applied in chemical instruments and methods, biochemical equipment and methods, measurement devices, etc., can solve the problem that the catalytic activity and structural integrity of protease are easily affected by environmental factors, and the physiological state of the sample donor is unavoidable. It can be easily unified and standardized, easy to popularize, and moderately stable.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
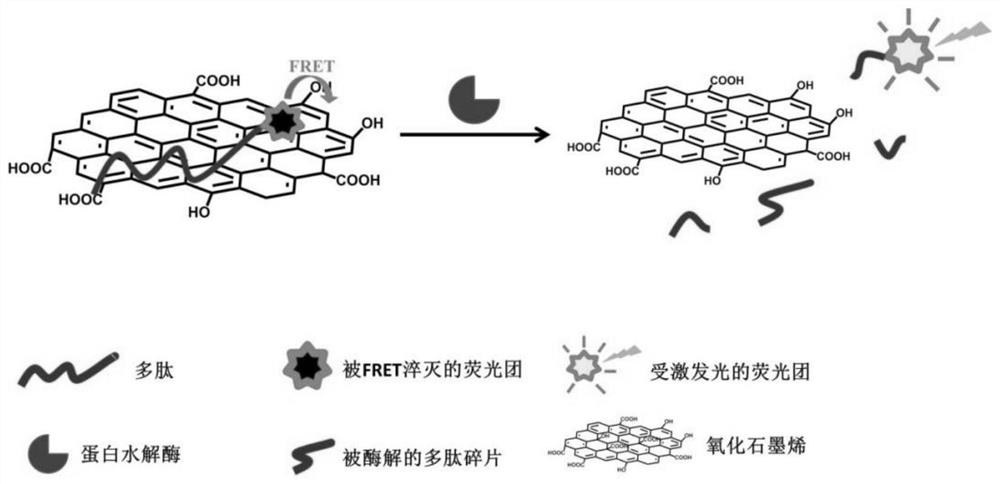
[0048] Feasibility Analysis of Plasma Protease Activity Measurement by Addition of Graphene Oxide (GO) and Fluorescently Labeled Peptides. Proceed as follows:
[0049] 1) GO was prepared into a 5 mg / mL solution with pure water. The fluorescently labeled polypeptide is: a peptide segment DKSKLKKTETQEKNPLP (SEQ ID NO: 2) labeled at the N-terminus by the fluorescent group MCA. Fluorescence-labeled polypeptide was prepared into a 5 mg / mL solution with pH 7.4 phosphate buffer solution (PBS).
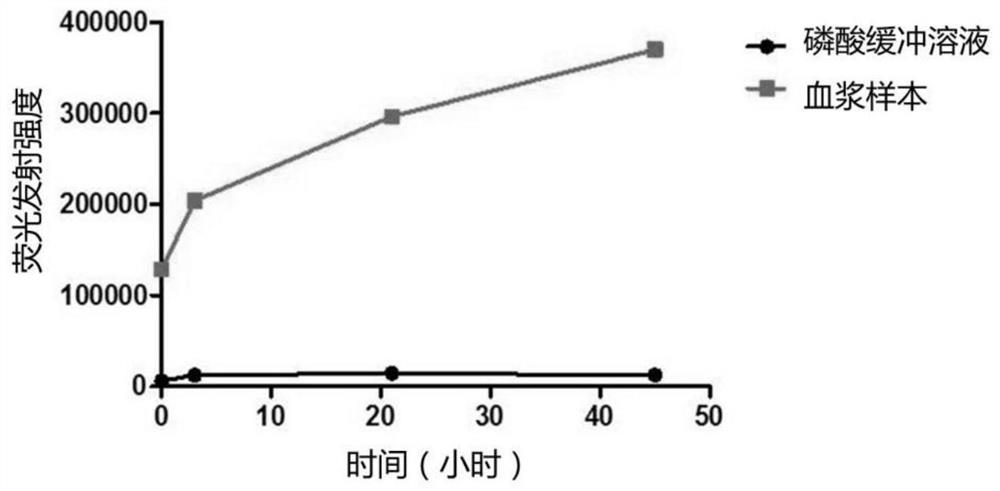
[0050] 2) Take 110 μL of plasma sample from a healthy person, add 2.3 μL of GO solution and 2.3 μL of fluorescent peptide solution. At the same time, take 110 μL of PBS, add 2.3 μL of GO solution and 2.3 μL of fluorescent peptide solution, as a negative control. The above solutions were all placed in a fluorescent 96-well plate.
[0051] 3) Immediately put the 96-well plate into a multifunctional microplate reader (Perkin Elmer Company, model EnSpire), incubate at 37°C, and start monitori...
Embodiment 2
[0055] To verify the response of protease activity in plasma to plasma sample storage temperature. Proceed as follows:
[0056] 1) Take five fresh plasma samples and mix equal volumes into one pooled plasma sample.
[0057] 2) Take three 1.5mL EP tubes, add 150μL of mixed plasma, and mark them as samples 1, 2, and 3 respectively. Sample No. 1 was placed in a constant temperature heater at 25°C; Sample No. 2 was placed in a refrigerator at 4°C; Sample No. 3 was placed in an ultra-low temperature refrigerator at -80°C. The storage time is 24 hours.
[0058] 3) GO was prepared into a 5 mg / mL solution with water. The fluorescently labeled polypeptide is: a peptide segment DKSKLKKTETQEKNPLP (SEQ ID NO: 2) labeled at the N-terminus by the fluorescent group MCA. Fluorescence-labeled polypeptide was prepared into a 5 mg / mL solution with pH 7.4 phosphate buffer solution (PBS).
[0059] 4) After the three plasma samples were stored for 24 hours, 3 μL of GO and fluorescent peptide s...
Embodiment 3
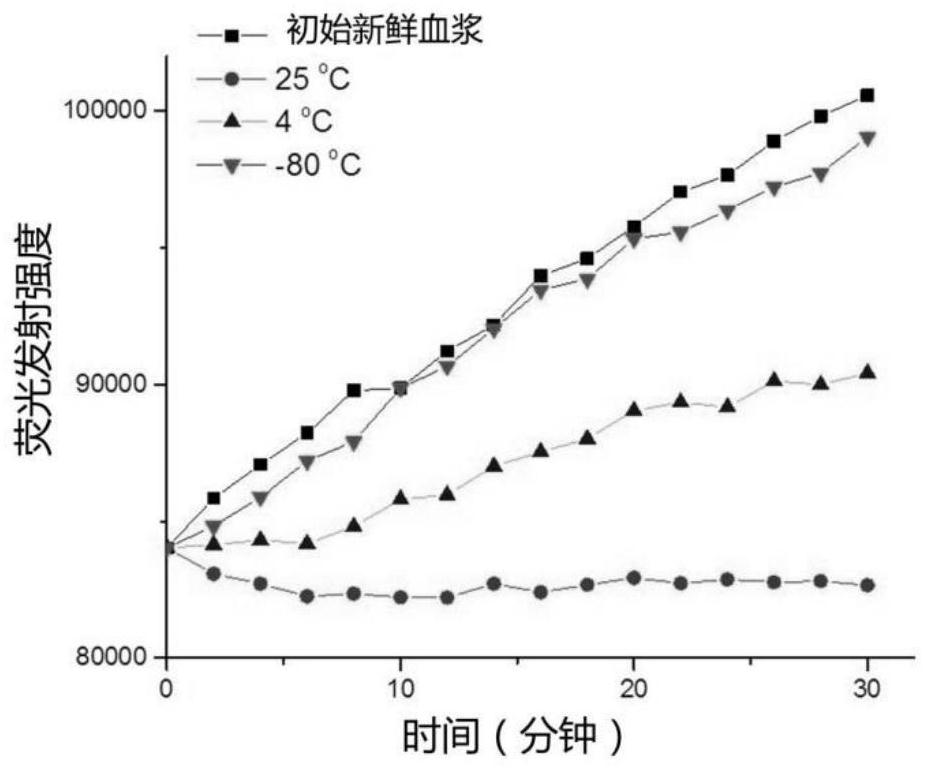
[0063] To verify the response of protease activity in plasma to plasma sample storage temperature and storage time. Proceed as follows:
[0064] 1) Take five fresh plasma samples and mix equal volumes into one pooled plasma sample.
[0065] 2) Take ten 1.5mL EP tubes, add 150μL of mixed plasma, and mark them as samples 0, 1, 2, 3, 4, 5, 6, 7, 8, and 9 respectively. Among them, samples No. 1-3 were placed in a constant temperature heater at 25°C; samples No. 4-6 were placed in a refrigerator at 4°C; samples No. 7-9 were placed in an ultra-low temperature refrigerator at -80°C. Storage time: samples 1, 4, and 7 were stored for 24 hours; samples 2, 5, and 8 were stored for 48 hours; samples 3, 6, and 9 were stored for 6 days. Sample No. 0 was directly tested in a fresh state without storage.
[0066] 3) GO was prepared into a 5 mg / mL solution with water. The fluorescently labeled polypeptide is: the peptide segment DKSKLKKTETQEKNPLP (SEQ ID NO: 2) labeled with the fluorescent g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com