Antibacterial medical isolation gown and manufacturing method thereof
A technology of isolation gown and antibacterial film, which is applied in the field of medical equipment and can solve the problems of reduced antibacterial effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
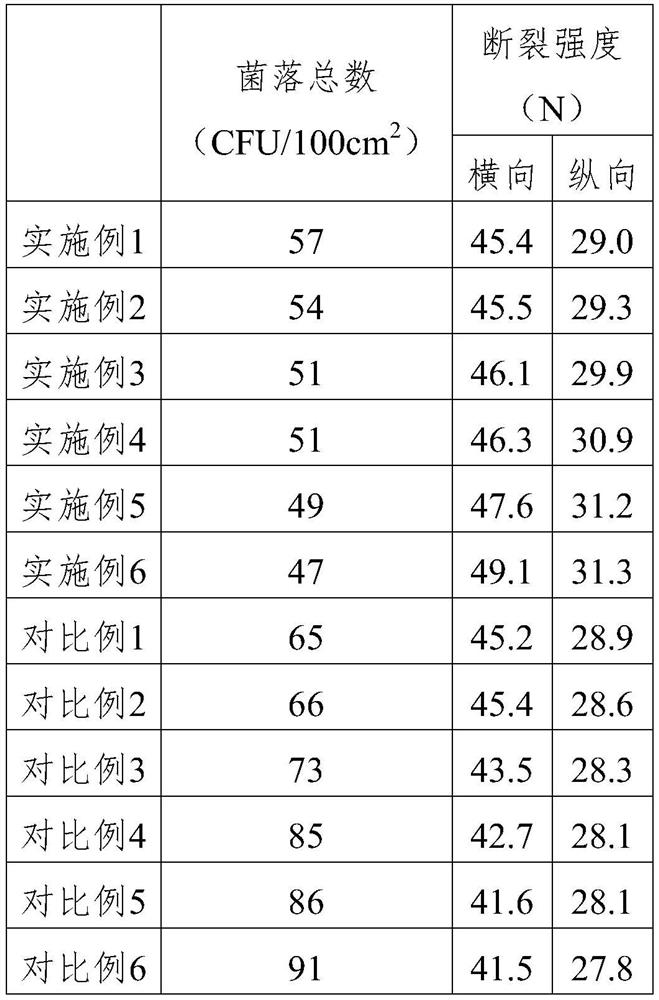
[0020] The present invention is an antibacterial medical isolation gown, comprising at least one outer non-woven fabric layer, a melt-blown nanofiltration layer and at least one inner non-woven fabric layer sequentially arranged from outside to inside, the outer non-woven fabric layer The cloth layer and the inner non-woven fabric layer comprise the following raw materials in parts by weight: 95 parts of polypropylene (PP), 15 parts of metallocene polypropylene (LMPP), 15 parts of maleic acid grafted LMPP, 8 parts of polylysine The molecular weight of the polylysine is 3000-4000, 2 parts of sodium benzoate, 3 parts of hyaluronic acid with a molecular weight of 1 million, 1 part of antioxidant, 1 part of nucleating agent, and 0.5 part of calcium stearate.
[0021] The antioxidant is a phosphite antioxidant.
[0022] The nucleating agent is an organic phosphate nucleating agent, NA-21.
[0023] The manufacture method of above-mentioned antibacterial medical isolation clothing, ...
Embodiment 2
[0028] The difference between Example 2 and Example 1 is that the outer nonwoven layer and the inner nonwoven layer contain the following raw materials in parts by weight: 90 parts of polypropylene (PP), 10 parts of metallocene polypropylene (LMPP) , 10 parts of maleic acid grafted LMPP, 5 parts of polylysine, 1 part of zinc oxide, 1 part of hyaluronic acid, 0.1 part of antioxidant, 0.1 part of nucleating agent, 0.1 part of calcium stearate.
Embodiment 3
[0030] The difference between Example 3 and Example 1 is that the outer nonwoven layer and the inner nonwoven layer contain the following raw materials in parts by weight: 100 parts of polypropylene (PP), 20 parts of metallocene polypropylene (LMPP) , 20 parts of maleic acid grafted LMPP, 10 parts of polylysine, 3 parts of silver ion antibacterial agent, 5 parts of hyaluronic acid, 2 parts of antioxidant, 2 parts of nucleating agent, 1 part of calcium stearate .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com