Application of 1,2,4triazolo4,3-bpyridazine derivatives in the preparation of anti-new coronavirus drugs
A pyridazine derivative and 3-B technology, applied in the field of medicine, can solve the problems of unbearable for patients, high cost, lack of curative effect in severe patients, etc., achieve wide application prospects, and suppress the effect of inflammatory factor storm
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Example 1 Inhibitory effect of let-7 on SARS-CoV-2
[0084] The inventor's team predicted the potential miRNA action sites on SARS-CoV-2 through bioinformatics, screened out the target miRNA, and verified the effect of the target miRNA on SARS-CoV-2 by western blotting.
[0085] Bioinformatics prediction of miRNA and target gene interactions: Download the SARS-CoV-2 genome sequence from NCBI, and use RegRNA software to predict the potential miRNA action sites on SARS-CoV-2 online. The results indicated that let-7 has potential binding sites on the S and M genes of SARS-CoV-2 organisms.
[0086] To verify the interaction between miRNA and target genes: HEK293T cells were co-transfected with S or M protein expression plasmids and miRNA expression plasmids. 48h after transfection, cells were lysed for western blot analysis of protein expression levels.
[0087] Western blotting: After cells were lysed with lysis buffer containing SDS and mercaptoethanol, they were boiled ...
Embodiment 2
[0094] Example 2 Inhibitory effect of C1632 on SARS-CoV-2
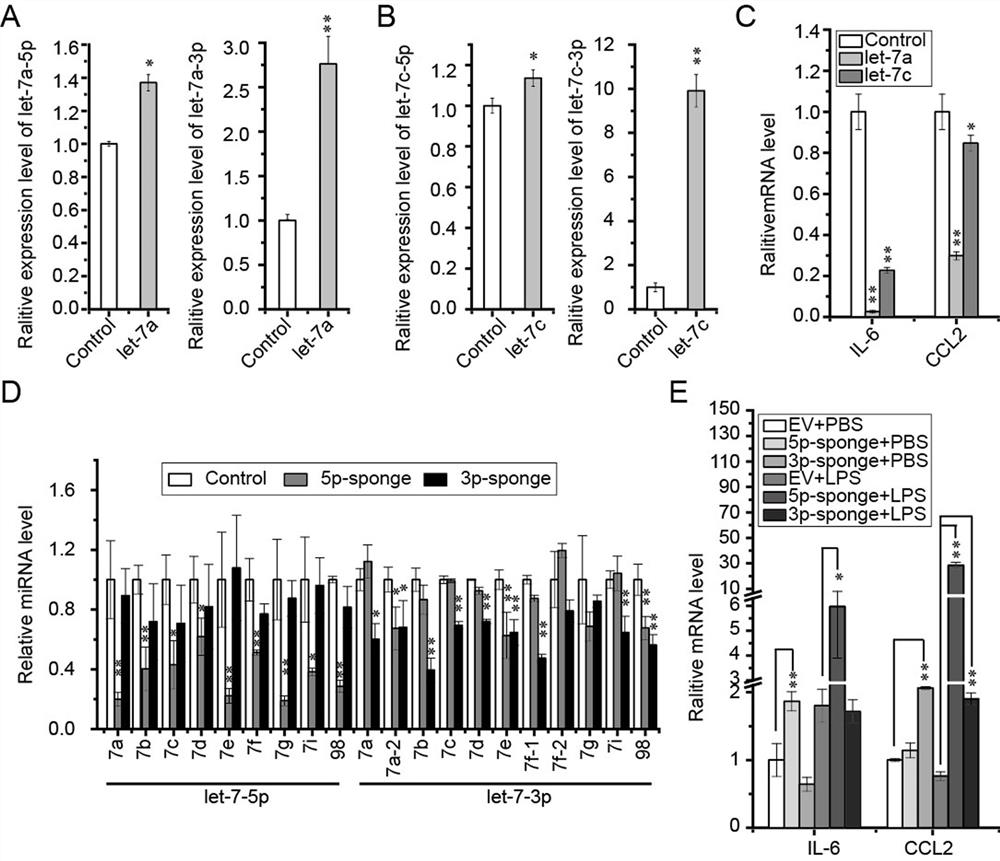
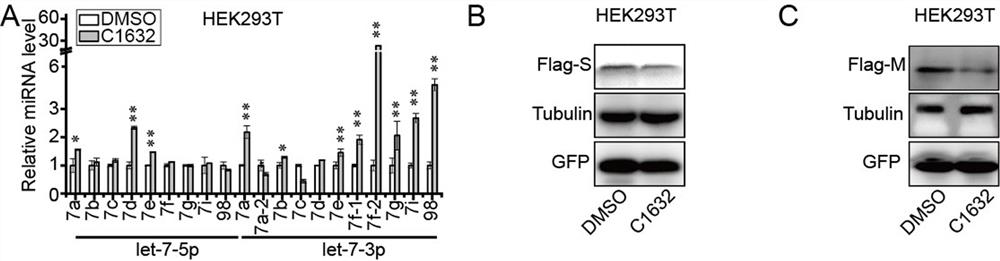
[0095] C1632 is a let-7 agonist. In order to determine whether C1632 can inhibit the expression of S and M proteins of SARS-CoV-2 by regulating the level of let-7 expression in cells, the inventors treated cells with C1632 for 2 days and detected let-7 by QRT-PCR. -7 expression level.
[0096] The result is as image 3 shown. The results showed that C1632 treatment made let-7a-5p, let-7d-5p, let-7e-5p, let-7a-3p, let-7b-3p, let-7e-3p, let-7f-1 in HEK293T cells , let-7f-2, let-7g, let-7i and miR-98 were significantly upregulated ( image 3 middle A). Western blot showed that C1632 could significantly inhibit the exogenous S protein in HEK293T cells ( image 3 B) and M proteins ( image 3 expression in C).
[0097] In order to explore whether the let-7 agonist C1632 also has the function of regulating inflammatory factors, the effect of C1632 on the expression levels of let-7 and inflammatory factors in HEK293T, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com