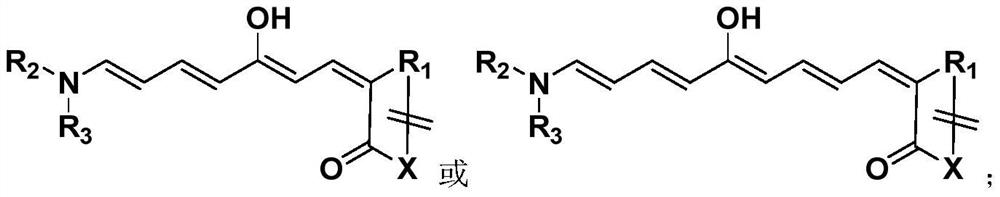
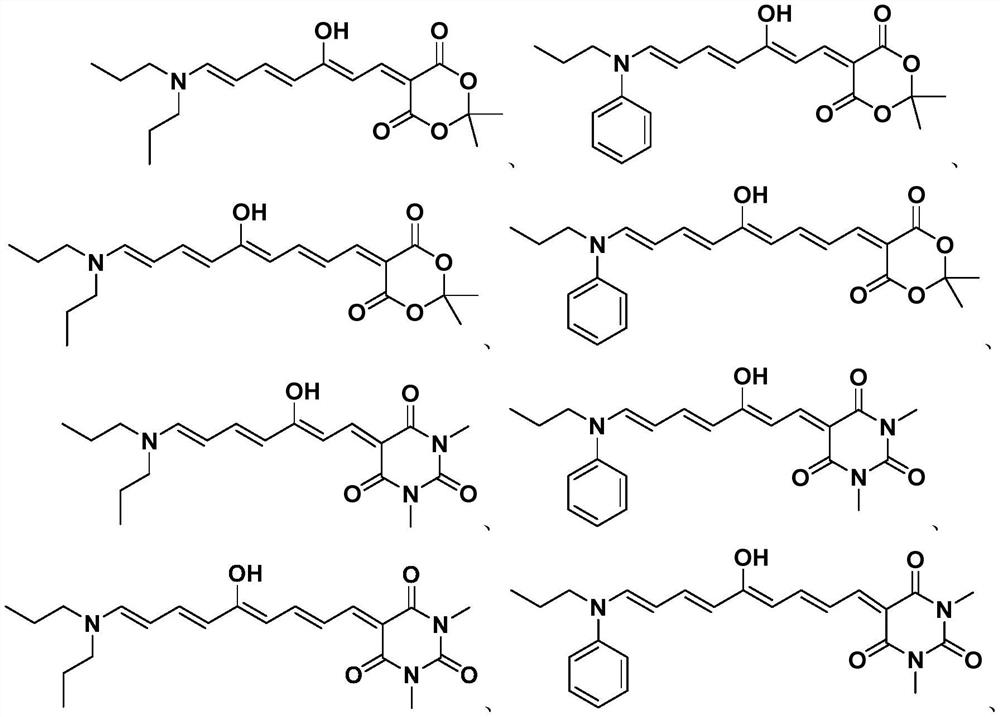
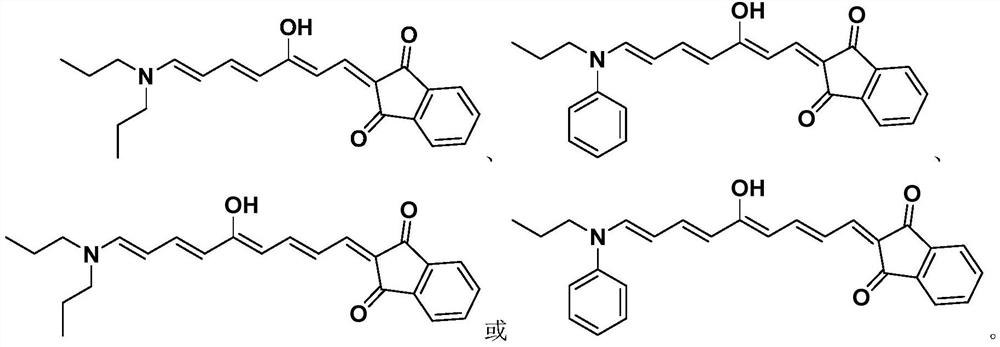
Polyene structure-containing donor-acceptor Stenhouse adduct and preparation method thereof
A technology of adducts and acceptors, which is applied in the field of donor-acceptor Steinhaus adducts containing polyene structures and its preparation, can solve the problems of high toxicity, low efficiency, and high cost, and achieve low cost, The effect of simple and convenient operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] This Example 1 provides the donor-acceptor Steinhaus adduct DASA-1 containing a polyene structure and its preparation method, and its synthesis schematic and synthesis steps are as follows:
[0042]
[0043] Step (1): Add 3-(2-furyl)acrolein (24.4mg, 0.2mmol), pyridine (0.8mg, 0.01mmol) and toluene (2.0mL) into a dry reactor, and stir at room temperature for 40 minutes , to obtain a homogeneous reaction system;
[0044] Step (2): Add Michaelis acid (57.6mg, 0.4mmol) into the reaction system obtained in step (1), and react at 60°C for 18 hours. After the reaction was completed, it was cooled to room temperature, and the solvent was removed under reduced pressure. The residue was separated and purified by silica gel column chromatography, and dried in vacuo to obtain an intermediate product (47.1 mg, yield 95%);
[0045] Step (3): Add the intermediate product (37.2 mg, 0.15 mmol), dipropylamine (15.2 mg, 0.15 mmol) and dichloromethane (2.0 mL) obtained in step (2) to ...
Embodiment 2
[0048] This Example 2 provides a donor-acceptor Steinhaus adduct DASA-2 containing a polyene structure and its preparation method, and its synthesis schematic and synthesis steps are as follows:
[0049]
[0050] Step (1): Add 3-(2-furyl)acrolein (24.4mg, 0.2mmol), pyridine (0.8mg, 0.01mmol) and toluene (2.0mL) into a dry reactor, and stir at room temperature for 30 minutes , to obtain a homogeneous reaction system;
[0051] Step (2): Add Michaelis acid (57.6mg, 0.4mmol) into the reaction system obtained in step (1), and react at 65°C for 20 hours. After the reaction was completed, it was cooled to room temperature, and the solvent was removed under reduced pressure. The residue was separated and purified by silica gel column chromatography, and dried in vacuo to obtain an intermediate product (46.6 mg, yield 94%);
[0052] Step (3): Add the intermediate product (37.2mg, 0.15mmol), N-propylaniline (20.1mg, 0.15mmol) and dichloromethane (2.0mL) obtained in step (2) to the dry...
Embodiment 3
[0055] This Example 3 provides a donor-acceptor Steinhaus adduct DASA-3 containing a polyene structure and its preparation method, and its synthesis schematic and synthesis steps are as follows:
[0056]
[0057] Step (1): Add 5-(furan-2-yl)penta-2,4-diene (29.6 mg, 0.2 mmol), pyridine (0.8 mg, 0.01 mmol) and toluene (2.0 mL) into a dry reactor ), stirred at room temperature for 40 minutes to obtain a homogeneous reaction system;
[0058] Step (2): Add Michaelis acid (57.6mg, 0.4mmol) into the reaction system obtained in step (1), and react at 60°C for 18 hours. After the reaction was completed, it was cooled to room temperature, and the solvent was removed under reduced pressure. The residue was separated and purified by silica gel column chromatography, and dried in vacuo to obtain an intermediate product (49.9 mg, yield 91%);
[0059] Step (3): Add the intermediate product (41.1 mg, 0.15 mmol), dipropylamine (15.2 mg, 0.15 mmol) and dichloromethane (2.0 mL) obtained in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com